
Chrozophora oblongifolia / شرشبان
Chrozophora obliqua sensu Muell. Arg. Croton oblongifolium Del. Chrozophora tinctoria (L.) A. Juss.
Tannoum, Abeera, Neela, Gabeera, Neele, Reen.
Chrozophora
Tannon, Sharshaban, Sharshar, qabra, Nawela
Euphorbiaceae

Aerial parts

Herbarium specimen
Ethnobotanical Characteristics
Description
An erect shrub, subshrub or woody herb up to 1 m high, evenly stellate and pubescent. Petioles 0.5-3.5 cm long. Leaf-blades triangular-ovate to triangular-lanceolate, 4-7 x 1.5-3 cm, acute or obtuse at the apex, cuneate, rounded or sub truncate at the base, subentire to repand-dentate, (3-5) nerved from the base, nerves impressed above and prominent beneath, evenly pubescent above and beneath. Stipules subulate, 2 mm long. Inflorescences up to 1.5 cm long, supra-axillary or leaf-opposed. Male flowers subsessile; sepals lanceolate to ovate-lanceolate, 3.5 mm long, stellate-pubescent. petals lanceolate to ovate-lanceolate, 4 mm long, lepidote without, yellowish; disc c. 1 mm diam.; stamens 4-7 (-12), variously connate, anthers sub-biseriate. Female flowers: pedicels 5 mm long, extending to 2 cm in fruit and becoming deflexed, 1-3 on a short peduncle; sepals and petals linear-lanceolate or lanceolate, otherwise as in the sepals; ovary 2 mm diam., densely silvery-lepidote; styles stellate-pubescent and papillose, bipartite, 1-2 mm long. Fruit rounded-trilobate, 5-6 x 7-9 mm, somewhat muricate, lepidote, bluish-purple. Seeds triangular-ovoid, 4-5 x 3-4 mm, coarsely tuberculate, yellowish-grey (Jongbloed et al. 2003, eFloras, Mandaville, 1990).
Habitat and Distribution
In sandy loam and gravelly sand. North and Tropical East Africa, Sinai, Arabian Peninsula, Pakistan to North West and India. The plant is wide spread in U.A.E. Scattered locations in the Hajar Mountains and on the east coast.
Part(s) Used
whole plant
Traditional and Medicinal Uses
The plant is used by traditional medicine practitioners as an antimicrobial, cathartic, emetic, and the immature fruits are used in the treatment of anal fissures, ulcers and blisters, burns (El-Ghonemy, 1993). The plant is used in Egypt in folk medicines for its hypoglycemic properties. In Sudan, stem or leaf extract used to treat gonorrhea (Batanouny, 1999). Chrozophora tinctoria is a dye plant native to Syria used as an illuminator pigment and as a food coloring. It is sold commercially in Europe for staining rugs.
Pharmacognosy and Phytochemistry
Plant material of interest
Dried leaf
General appearance
It is light brown in colour, thick, cordate with crenate or sinuate margin. It has prominent venation at the lower surface, which also bears bristles. The upper epidermis shows dark brown spots and small cavities.
Microscopical characteristics
The leaf is dorsiventral. Cuticle is not detected. Both upper and lower epidermises consist of polygonal cells of different outlines and sizes. The paracytic stomata are fairly distributed in both epidermises. Both epidermises embed numerous stellate covering trichomes with the member individual trichomes either having sharp or rounded blunt tips. The underlying layer of mesophyll palisade layer consists of long straight narrow parenchyma cells yellowish in colour and each is rich in cellular contents. The palisade layer is underlain by many layers of spongy mesophyll parenchyma cells with irregular shapes. Some cells contain a single cluster crystal of calcium oxalate each; other cells contain starch grains and other contents. Spongy cells embed vascular tissues that include short tracheids with pitted cell walls and relatively long vessels, which are spirally thickened. The spongy layer just above the lower epidermis resembles that of the palisade layer but cells are shorter and broad and they are colourless.
Powdered plant material
The leaf powder is a brownish-yellow fine homogeneous powder slightly aromatic with an acrid taste. Microscopically, the powder shows numerous detached stellate covering trichomes and yellowish-green leaf fragments exhibiting palisade and spongy mesophyll parenchyma cells with their contents of particles and crystalline masses, particularly calcium oxalate rosettes, in addition to dark grey adjacent spirally thickened vessels. The powder also shows groups of calcium oxalate rosettes, free or inside mesophyll parenchyma cells.
Parts studied
leaf
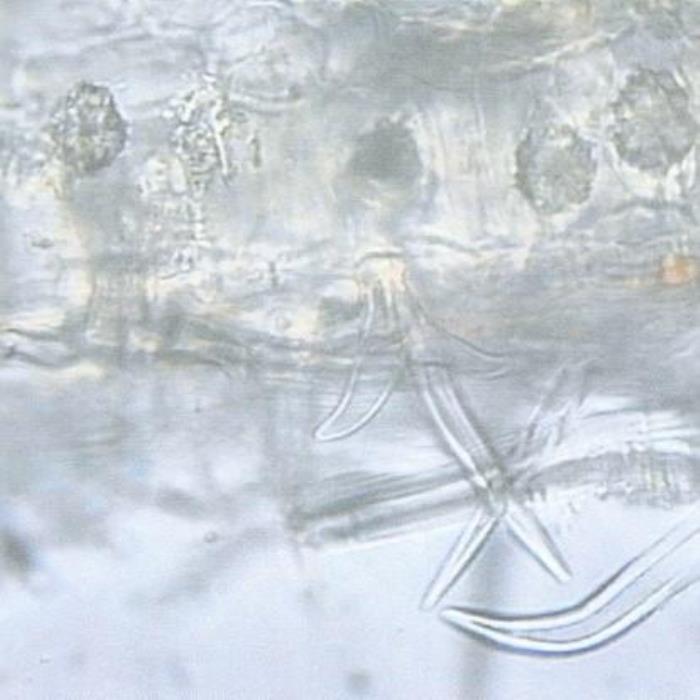
A) TS of leaf
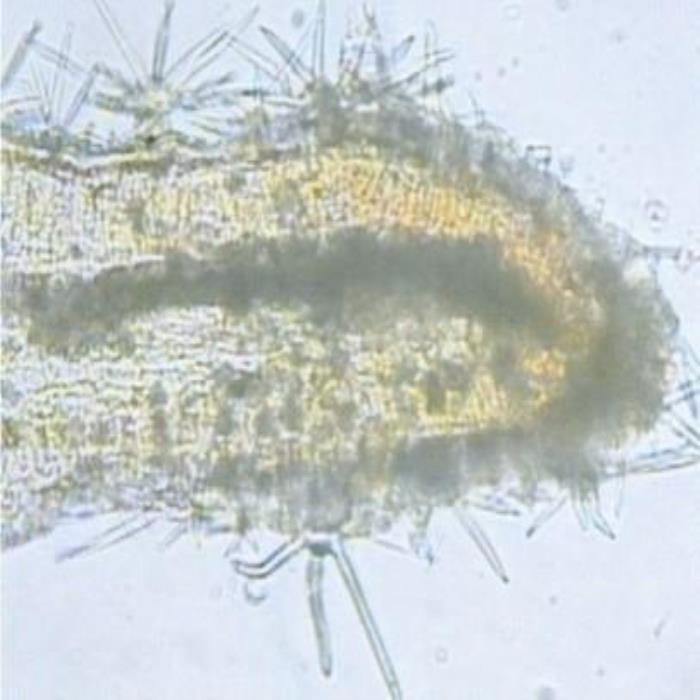
B) TS at leaf margin
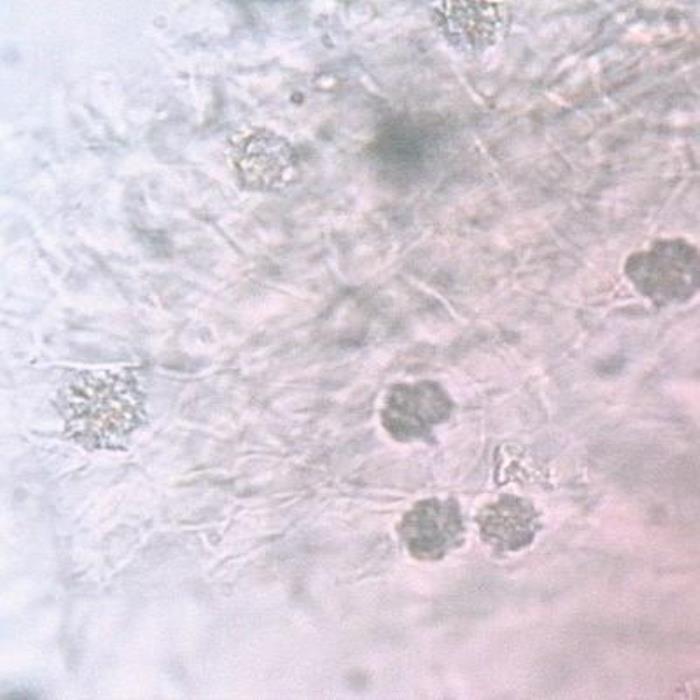
C) Spongy mesophyll
- A. Leaf TS. The lower epidermis showing the attachment of a stellate covering trichome, part of the mesophyll whose parenchyma cells partly enclosing cluster crystals (rosettes) of an oxalate.
- B. TS at the margin (leaf). Stellate covering trichomes densely scattered on both epidermises. Cluster crystals of calcium oxalate are scattered in the spongy mesophyll.
- C. The spongy mesophyll parenchyma cells; some cells contain large single cluster crystal of calcium oxalate.
Chemical constituents
Flavonoids, tannins, sterols and /or triterpenes and volatile bases (Al-Yahya M.A., et.al 1990). Contents of aerial parts: phenylpropanoid glucosides: 4-O-methyl guaiacylglycerol 9-O-beta-glucopyranoside, 8-O-beta-glucopyranoside, syringin, benzyl alcohol glucoside, isorhamnetin-3-O-beta-glucopyranoside-7-O-alpha-rhamnopyranoside and quercetin-3-O-beta-glucopyranoside-7-O-alpha-rhamnopyranoside (Mohamed, 2001). Aerial parts also contain dolabellane diterpenoids (Khaled, 1995).
The following chemical studies have been carried out (Quality Control methods, 1998; Evans, 1996) on the plant Chrozophora oblongifolia (ZCHRTM unpublished work):
Physicochemical Constants
Loss of weight on drying at 105°C: 9.00
Absolute alcohol solubility: 2.40
Water solubility: 20.80
Successive extractives (%)
Petroleum ether (60-80) °C : 1.70
Chloroform : 1.25
Absolute alcohol : 7.15
Water soluble : 16.8
Ash values (%)
Total ash : 7.00
Water soluble ash : 2.00
Acid insoluble ash (10% HCl) : Nil
pH values (aqueous solution)
pH of 1% solution : 5.234-5.235
pH of 10% solution : 5.087-5.099
Elemental analyses
Ash values (British Herbal pharmacopeia)
Assay and identification of metal (AOAC international)
|
Apparatus |
AA-6800 Shimadzu-Flame method |
||||
|
Element |
Std. conc. µg/ml(ppm) |
Sample conc.mg/ml |
Samples absorbance |
Actual conc.mg/ml |
Actual conc. (%) |
|
Cr |
1, 2, 4 |
20 |
0.0109 |
0.016650 |
0.0016650 |
|
Zn |
0.25, 0.5, 1 |
20 |
0.01440 |
0.0070250 |
0.0007025 |
|
Cu |
1, 2, 4 |
20 |
0.0277 |
0.00783 |
0.000783 |
|
Fe |
1, 2, 4 |
20 |
0.7497 |
0.31557 |
0.031557 |
|
Ca |
5, 10, 20 |
1 |
0.0603 |
11.312 |
1.1312 |
|
Pb |
1, 2, 4 |
20 |
0.0012 |
0.0018050 |
0.0001805 |
|
Cd |
0.25, 0.5, 1 |
20 |
0.0043 |
0.0002 |
0.00002 |
|
K |
1, 2, 4 |
1 |
0.857 |
7.712 |
0.7712 |
UV Spectral studies
|
Ultraviolet Spectrum (USP) |
||||
|
Apparatus |
Milton Roy Spectronic Genesys 5 Spectrophotometer - Milton Roy. |
|||
| Sample conc. (mg / ml) | Solvent | λ max (nm) | λ min (nm) | Abs.( λ max - λ min) |
|
2.051375 |
Intestinal Fluid simulated without pancreatic pH=7.50.1 |
268 |
- |
0.815 |
| 0.10025 | Gastric Fluid simulated without pepsin pH =1.20.1 | 268 | 250 | 0.893 - 0.765 |
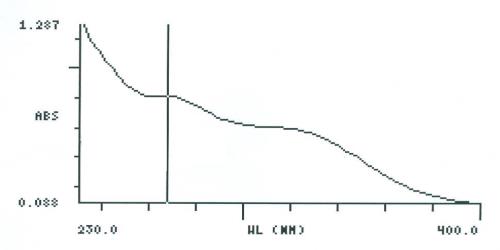
Intestinal Fluid simulated without pancreatic
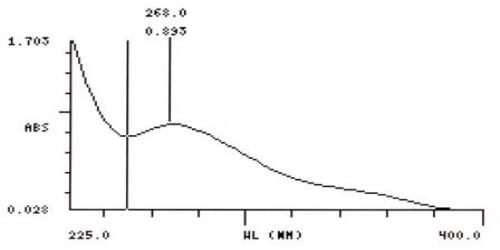
Gastric Fluid simulated without pepsin
Chromatographic Studies
Thin layer chromatography (Wagner and Bladt, 1996)

A

B

C
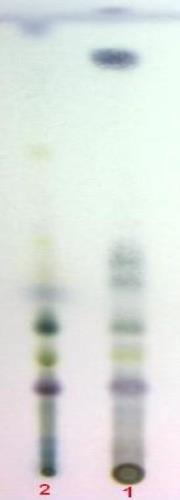
D
TLC fingerprint of Pet. ether (60-80) °C extract (track 1) and MeOH extract (track 2)
|
Mobile phase Fig. |
A&B |
: |
Ethyl acetate, methanol, water (100:13.5:10) |
|
|
C |
: |
Toluene, ethyl formate, formic acid (54:1) |
|
|
D |
: |
Toluene, ethyl acetate (93:7) |
|
Detection |
A |
: |
UV 254nm |
|
|
C |
: |
UV 365nm |
|
Derivatization |
B&D |
: |
Vanillin-Sulphuric acid-vis |
Pharmacological and Toxicological studies
Aqueous extract
The toxicity of Chrozophora obliqua was evaluated in rats fed at 2% or 10% of the standard diet. When compared with controls, body weight gains and feed efficiency were adversely affected by both treatments. Although the rats fed 10% Chrozophora sp. diet had the lowest growth rate, bouts of soft feces and entero and hepato-nephropathy, no death occurred among the rats. These changes were accompanied by increases in serum Gamma-glutamyltransferase (GGT) and Aspartate aminotransferase (AST) activities, and urea and cholesterol concentrations. However, the total protein and albumin levels, macrocytic hypochromic anaemia and leucopenia decreased (Adam et al., 1999). It was reported that Chrozophora tinctoria extract was useful for treating mice skin carcinogenesis (Rezazadeh, 2004).
Antioxidant activity of methanol extract of Chrozophora tinctoria has been reported (Talischi, 2005). The analogue of brevifolin carboxylic acid and its methyl ester were isolated and identified from the aqueous ethanolic extract of the leaves of Chrzophora sp. and the antioxidant activity of the new compounds was determined by checking the scavenging activity against 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical (Hawas, 2007).
The effect of Chrozophora plicata on Nubian goats and desert sheep were investigated. The main signs of Chrozophora poisoning in both species of ruminants were salivation, dyspnea, bloat, inappetence, dullness, diarrhea, paresis of the hind limbs, decumbency and lateral deviation of the head and neck. The main lesions were hemorrhage in the lungs, heart and kidneys, pulmonary cyanosis and edema, hepatic fatty change and depletion of glycogen, catarrhal enteritis, ascites, hydropericardium and serous atrophy of the cardiac fat and renal pelvis (Gala, 1988). An increase in the concentration of urea, ammonia and bilirubin and in the activity of GOT and a decrease in total protein were detected in the serum. Hematological changes indicated the development of anemia (Galal, 1988).
The in vitro antimicrobial activities of the whole plant extract of Chrozophora senegalensis and its fractions (ethyl acetate-EAA, n-butanol-NBE,) were assayed using the agar plate diffusion and nutrient broth dilution methods were tested on Bacillus subtilis (NCTC 8326 B76), Escherichia coli (ATCC 11775), Pseudomonas aeruginosa (ATCC 10145), Staphylococcus aureus (ATCC 021001). Aspergillus flavus, Aspergillus niger, Candida albicans and Salmonella typhi - laboratory isolates. CEE, EAA and NBE inhibited all the test bacterial organisms and the fungus Aspergillus flavus. (Usman, 2007).
The antiplasmodial activity of crude extracts from the leaves and the stems of Chrozophora senegalensis and Chrozophora sp. extracts was determined in-vivo (Benoit, 2008).
A significant contractile response, which was antagonized by atropine, was produced by ethanolic extract of Chrozophora sp. on isolated guinea pig ileum. No effect of both ethanolic and chloroform extracts was observed on isolated frogs rectus abdominus, nor did they modify the effect of acetylcholine on the muscle. An appreciable fall in blood pressure of anaesthetized rabbits was produced by both extracts.
The following pharmacological and safety evaluation studies were carried out on the aqueous extract of plant Chrozophora oblongifolia at ZCHRTM labs. (Derelanko 2002; Han, 2003):
|
ACTIVITY |
RESULTS |
|||
|
Strong |
Moderate |
Mild |
Negative |
|
|
Anti-stress ( swim test) |
√ |
|
|
|
|
Anti-inflammatory |
|
|
|
√ |
|
Effect on Guinea pig ileum |
√ |
|
|
|
|
Anti-hemorrhoids |
|
√ |
|
|
|
Acute toxicity < 5g/kg |
|
|
|
√ |
| Motor in-coordination (grip strength & motor activity | √ | |||
|
Rectal temperature |
|
|
|
√ |
|
Locomotor |
√ |
|
|
|
|
Body weight |
|
|
|
√ |
|
Vital organs |
|
|
|
√ |
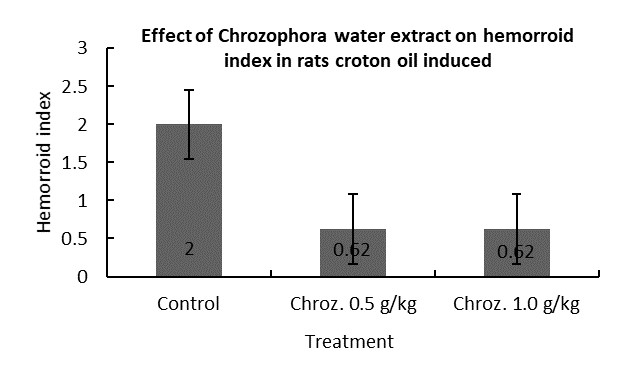
Effect on hemorrhoid index
Summary of Results
The acute studies demonstrated that a plant extract is safe and did not cause any detrimental effects in vivo under the conditions investigated in this study. In the acute study the plant extract caused no mortality up to a maximum practical dosage of 5g/kg body weight. However, it may be concluded that plant extract does not produce any significant acute and cumulative toxicity at the doses administered, based on the various parameters investigated.
Antimicrobial activity
The aqueous extract of the whole plant was tested against Mycobacterium smegmatis, C. tropicalis, different strains of Methicillin Resistant Staphylococcus aureus, different strains of ESBL-producing K. pneumonia, E. coli, Pseudomonas aeruginosa and showed no inhibition of growth.
References
- Adam SEI, Al Redhaiman K N, Al-Qarawi A A. (1999). Toxicity of Chrozophora obliqua in rats, Phytotherapy Research, 13: 630-632.
- Al-Yahya, M. A., Al-Meshal, I. A., Mossa, J. S., Al-Badr, A. A., & Tariq, M. (1990). Saudi plants: A phytochemical and Biological approach. King Abdul Aziz city for Sciences, 111.
- Batanouny, K. (1999). Wild medicinal plants in Egypt. Cairo, Gaza, Egypt: Dept. of Botany, University of Cairo.
- British Herbal Pharmacopoeia (1996).4th Ed.: British Herbal Medicine Association (BHMA).
- Bauer AW, Kirby WMM, Sheriss JC, Turck M. (1966) Antibiotic susceptibility testing by standardized single method. Am J Clin Pathol; 45:493–6.
- Benoit-Vical F, Soh PN, Saléry M, Harguem L, Poupat C, Nongonierma R. (2008). Evaluation of Senegalese plants used in malaria treatment: focus on Chrozophora senegalensis. J Ethnopharmacol, 116: 43-48.
- Derelanko, M. J., & Hollinger, M. A. (2002). Hand book of toxicology. (2nd ed.). Boca Raton, USA: CRC Press.
- Eddouks M, Lemhadri A, Michel JB. (2005). Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J Ethnopharmacol, 98: 345-350.
- El-Ghonemy, A.A. (1993). Encyclopedia of medicinal plants of the United Arab Emirates. (1st ed.). Abu Dhabi, UAE: United Arab Emirates University.
- Evans, W.C (1996).Trease and Evans’ Pharmacognosy,(14th ed,p.105 )Saunders ,London.
- Flora of Pakistan. e-floras, Web.
- Gadgoli C, Mishra SH. (1999). Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J Ethnopharmacol, 66: 187-192.
- Germano MP, De Pasquale R, D'Angelo V, Catania S, Silvari V, Costa C. (2002). Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem, 50: 1168-1171.
- Ghazanfar, S. A. (1994). Handbook of Arabian Medicinal Plants. Boca Raton, USA: CRC Press.
- Ghule BV, Murugananthan G, Nakhat PD, Yeole PG. (2006). Immunostimulant effects of Capparis zeylanica Linn. Leaves. J Ethnopharmacol, 108: 311-5.
- Ghule BV, Murugananthan G, Yeole PG. (2007). Analgesic and antipyretic effects of Capparis zeylanica leaves. Fitoterapia, 78: 365-369.
- Han , J., & Hoosier, G. L. V. J. (2003). Hand book of laboratory science, animal models. (Second ed., Vol. II). USA: CRC Press.
- Huseini HF, Alavian SM, Heshmat R, Heydari MR, Abolmaali K. (2005). The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomedicine, 12:619-624.
- Jongbloed, M., Feulner, G., Boer, B., & Western, A. (2003). The comprehensive guide to the wild flowers of the United Arab Emirates. Abu Dhabi, UAE: ERWDA.
- Mandaville, J. (1990). Flora of Eastern Saudi Arabia. London, UK.: Kegan Paul International Ltd.
- Mohamed, K. M., Ohtani , K., Kasai , R., & Yamasaki, K. (1995). 3-hydroxy-3-methylglutaryl dolabellane diterpenes from chrozophora obliqua. Phytochemistry, 39(1), 156-161. Retrieved from http://www.sciencedirect.com/science/article/pii/003194229400917I
Mohamed, K. (2001). Phenylpropanoid glucosides from Chrozophora oblique,Phytochemistry, 548(4),615-618. - Mothana R A A, Abdo SA ,Hasson S, Althawab FM, Alaghbari SA, Lindquist U.( 2008) Antimicrobial, antioxidant and cytotoxic activity and phytochemical screening of some Yemeni medicinal plants. Evid Based Complment Alternat Med. 7(3):323-30.
- Mothana R A A, Abdo SA ,Hasson S, Althawab FM, Alaghbari SA, Lindquist U.( 2008) Antimicrobial, antioxidant and cytotoxic activity and phytochemical screening of some Yemeni medicinal plants. Evid Based Complment Alternat Med. 7(3):323-30.
- Official Methods of Analysis of AOAC International (1999).16th. Ed.Vol.I and II Panico AM, Cardile V, Garufi F, Puglia C, Bonina F, Ronsisvalle G. (2005). Protective effect of Capparis spinosa on chondrocytes. Life Sci, 77:2479-288.
- Purohit A, Vyas KB. (2005). Hypolipidaemic efficacy of Capparis decidua fruit and shoot extracts in cholesterol fed rabbits. Indian J Exp Biol, 43: 863-866.
- Quality control methods for medicinal plant materials (1998).World Health Organization, Geneva.
- Tesoriere L, Butera D, Gentile C, Livrea MA. (2007). Bioactive components of Capparis spinosa L. from Sicily and antioxidant effects in a red meat simulated gastric digestion, J Agric Food Chem, 55: 8465-8471.
- Trombetta D, Occhiuto F, Perri D, Puglia C, Santagati NA, De Pasquale A, Saija A, Bonina F. (2005). Antiallergic and antihistaminic effect of two extracts of Capparis spinosa L. flowering buds. Phytother Res, 19: 29-33.
- Wagner, H. Bladt, S.(1996). Plant Drug Analysis-A Thin layer Chromatography Atlas, (2nd Ed.) Springer-Verlag, Berlin Heidelberg.
- Yadav P, Sarkar S, Bhatnagar D. (1997). Action of Capparis decidua against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res, 36: 221-228.
- ZCHRTM unpublished work
- محمد العودات, (1988), جورج لحام النباتات الطبية واستعمالاتها، الجزء الأول-الطبعة الثانية.الأهالي، سوريا
