
Arnebia hispidissima / كحيلة
Anchusa asperrima Delile Anchusa hispidissima Sieber ex Lehm. Arnebia asperrima (Del.) Hutchison & Dalz. Lithospermum hispidissimum Lehman Lithospermum vestitum Wall.
Atan, Atani, Fahtag, Aweanah Al Musalman, Arnebah
Arabian primrose, Prophet Flower
Kahilah, Arnebah
Boraginaceae

Aerial parts

Herbarium specimen
Ethnobotanical Characteristics
Description
Annual or perennial branched herb; stem prostrate to decumbent or suberect. Root exuding a purplish dye. Stem(s), branches and leaves hispid, with small (up to 0.6 mm long) weaker hairs. Basal leaves 15-35 x 3-4 mm (cauline shorter), lanceolate, entire, ± revolute, tapering towards the base. Inflorescence a terminal scorpioid cyme up to 11-18 cm long in fruit, bracteate; bracts 4-8 mm long, lanceolate. Flowers heterostyled. Calyx 5-partite, 6-9 mm long, hispid, lobes unequal, linear-lanceolate. Corolla vinous to the outside, tubular; tube in long styled flowers 8 mm long, infundibuliform, 4-5 mm broad; lobes broadly ovate, margin entire to undulate, spreading, throat without hairs. Tube pubescent within just below the anthers (in long styled flowers). Anthers 1.5 mm long. Ovary deeply 4-lobed. Style simple, slender. Long styles reaching throat of corolla, short styles 4-5 mm long. Stigmas 2, flabellate. Nutlets ± 1.7 mm long, ovoid, light brown, minutely tuberculate (Jongbloed 2003, Flora of Pakistan, Mandaville, 1990).
Habitat and Distribution
Throughout Arabia, in dry sandy areas. Also distributed in Northern and East Africa and Pakistan (Ghazanfar, 1994). The plant is wide spread in U.A.E, especially in the North Emirates and Northern region of Abu Dhabi Emirate. Also recorded from far west of Abu Dhabi Emirate and in the Ru’us al-jibal.
Part(s) Used
whole plant
Traditional and Medicinal Uses
The whole plant is used for treating fevers including malarial fever. The plant is boiled in water (to which sugar may be added) and taken as tea to reduce fever. The flowers and roots are used in cosmetics. The roots yield a purple-blue dye which is rubbed on the face as a cosmetic. The roots of Arnebia decumbens (kahal, kahla) produce a red dye, which is also used on the facial cosmetics (Ghazanfar, 1994, El-Ghonemy, 1993). The Paste of the roots is applied on inflamed injury (Pak. J. Bot., 2010)
Pharmacognosy and Phytochemistry
Plant material of interest
Flower, root and leaf
Leaf: It is small, yellowish-brown to dark brown in colour with white bristles giving a silvery tint; it is curled inwards and brittle.
Microscopical characteristics
Leaf: A transverse section of the leaf shows its dorsiventral character where the palisade tissue is found only below the upper epidermis. Both the upper and the lower epidermises consist of small polygonal cells. The upper epidermis is covered with a thin cuticle. Both epidermises imbed comparatively large warty covering trichomes with sharp pointing ends, and their bases are surrounded by characteristic epidermal cells with thick walls forming circular patterns. The stomata are oval and paracytic. Below the upper epidermis is one layer of palisade cells that consists of loosely packed oblong parenchyma cells very rich in chlorophyll and associated pigments (dark brown and yellowish-brown, which also impregnate the cell walls). The upper and lower epidermises contain only traces of colored materials. Below the palisade layer are the spongy parenchyma cells, which are also rich in colored pigments that are polygonal embedded in vascular tissues. The flower is small, yellowish-brown and brittle.
Root: A transverse section of the root from the periphery inwards shows the following characters: the epidermal layer with small rectangular cells, a multi-layered cortex consisting of small polygonal cells (all containing colored pigments); phloem tissues; xylem tissues; modular rays that form characteristic heart-shaped patterns; and pith with rounded parenchyma cells.
Powdered plant material
Leaf: The material consists of a slightly coarse heterogeneous powder, brown colored with white particles due to the presence of bristles; it is odorless but has a slight persistent bitter taste. Microscopically, the powder shows many small light brown leaf fragments especially, of the mesophyll region which are rich in yellowish-brown and dark brown colored pigments. It also shows many large detached warty unicellular trichomes with sharp pointed ends.
Flower: The material consists of a very fine homogeneous dark yellow powder with a pleasant aromatic odor and a slightly acrid taste. Under the microscope, the powder shows numerous detached sharp-pointed, conical unicellular warty trichomes, and also short coiled ones. It shows the fibrous layer of the floral anther, which is composed of small cells and rod-like thickenings together with some yellow 8-shaped pollen grains.
Root: The material consists of a light violet-brown coarse heterogeneous powder with a slight odour of dry straw and a slightly acrid taste. Microscopically, the powder shows violet-brown cork cells with distinct outlines and compactly packed grayish bordered pitted and articulately thickened vessels, together with large fragments of tissues of reddish-brown colored cortical cells.
Parts studied
Leaves, shoots, and roots
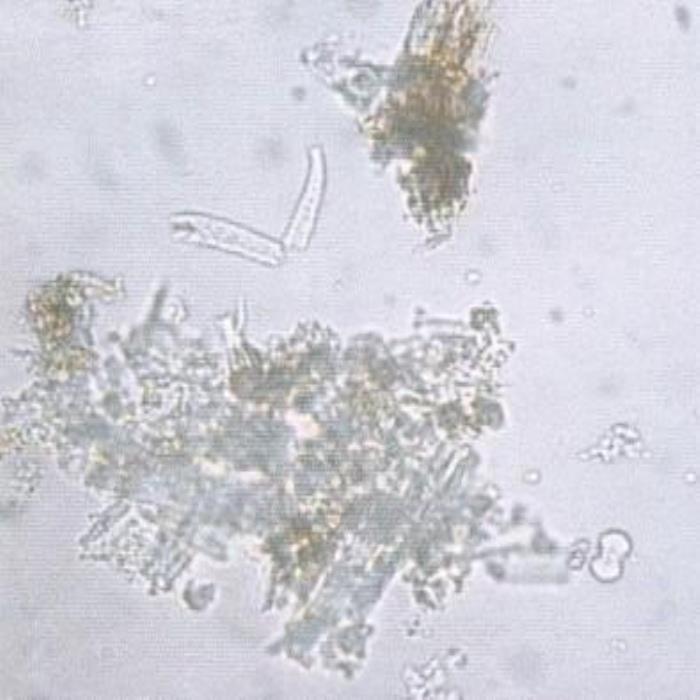
A) Different fragments
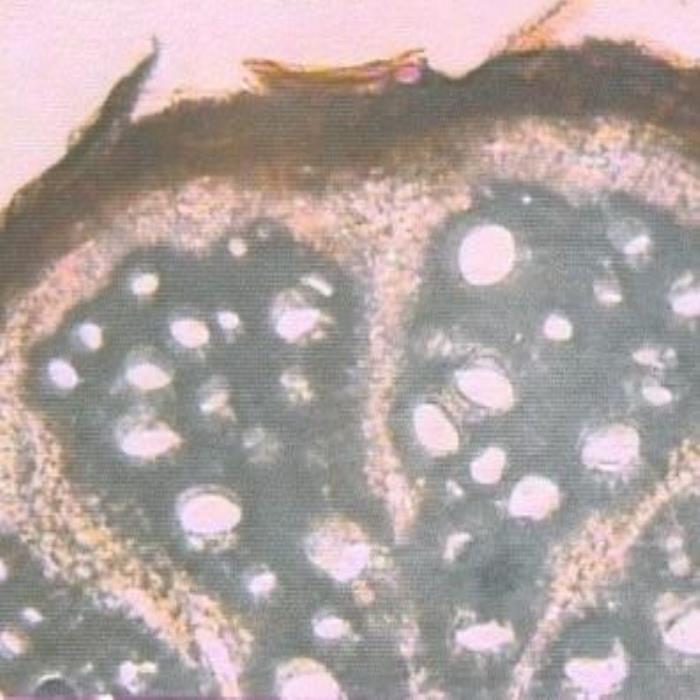
B) TS of root
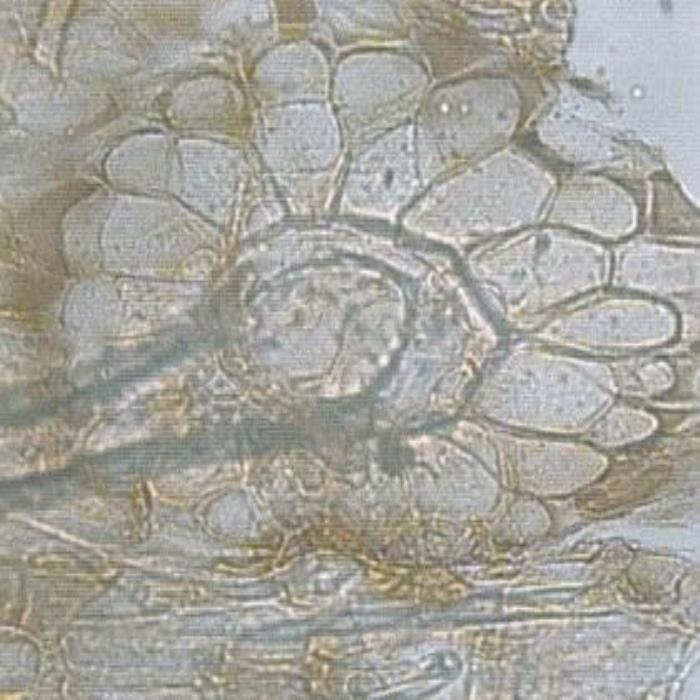
C) Large characteristic cells
- A. A group of different fragments showing broken warty covering trichomes, epidermal cells and mesophyll tissues containing colored pigments, vascular tissues and an 8-shaped pollen grain (leaf with little floral parts).
- B. A portion of a transverse section of the root showing the pattern of the vascular tissues (dark areas with light colored circular areas) and medullary rays. Shown also is the epidermal layer containing colored matter and the cortex zone (light pink) underneath.
- C. Large characteristic cells surrounding the base of a large covering trichome of the lower epidermis of leaf.
Chemical constituents
The roots were profiled for shikonin derivatives such as arnebin-5, arnebin-6, teracryl shikonin, arnebinone and acetyl shikonin, arnebin-7, alkannin acetate, alkannin isovalerate, alkanet and β-sitosterol, and alkannin β-hydroxyisovalerate (Singh,2003; Hamid A.,1983). A flavonoid, characterized as vitexin, has been isolated from the fresh flowers of Arnebia hispidissima (Rastogi, 1995). A dye, commonly known as Ratanjot in Indo-Pakistan medicine is obtained from the roots of A. hispidissima. Red pigment in the roots is composed of a group of naphthoquinonic: shikonin (and esters), its optical isomer alkannin (and derivatives) and their common racemic form shikalkin, as well as arnebifuranone. The aerial parts include arnebins and the triterpenoid betulin, β-amyrin and lupeol (Jansen, 2005). Triterpenes and β-Amyrin (0.29%) (Jain, 2003). In Roots: alkannin (also arnebin-4), acetyl alkannin or arnebin-3, isovaleryl alkannin, β-hydroxy isovalerylalkannin, shikonin, deoxy alkannin, deoxy shikonin, or arnebin-7 (Papageorgiou, 1999).
The following chemical studies have been carried out (Quality Control methods, 1998; Evans, (1996) on the plant Arnebia hispidissima (ZCHRTM unpublished work):
Physicochemical constants
Loss of weight on drying at 105°C: 10.40
Absolute alcohol solubility: 2.00
Water solubility: 17.8
Successive extractives (%)
Petroleum ether (60-80) °C : 1.40
Chloroform : 0.90
Absolute alcohol : 2.60
pH values (aqueous solution)
pH of 1% solution : 9.114-9.140
pH of 10% solution : 8.686-8.690
Ash values (%)
Total ash : 17.98-25.67
Water soluble ash : 5.00
Acid insoluble ash (10% HCl) : 1.47-2.00
Elemental analyses
Ash values (British Herbal Pharmacopeia)
Assay and identification of metal (AOAC International)
|
Apparatus |
(AA-6800 Shimadzu-Flame method) |
||||
|
Element |
Std. conc. µg/ml |
Sample conc.mg/ml |
sample absorbance |
Actual conc.mg/ml |
Actual conc. (%) |
|
Cr |
1, 2, 4 |
10 |
0.000 |
-Ve |
-Ve |
|
Zn |
0.25,0.5, 1 |
10 |
0.1074 |
0.00928 |
0.000928 |
|
Cu |
1, 2, 4 |
10 |
0.0234 |
0.01044 |
0.001044 |
|
Fe |
1, 2, 4 |
10 |
0.5588 |
0.39579 |
0.039579 |
|
Ca |
1, 2, 4 |
10 |
0.5883 |
3.5656599 |
0.35656599 |
|
Pb |
1, 2, 4 |
10 |
0.0172 |
0.00172 |
0.000172 |
|
Cd |
0.25, 0.5, 1 |
10 |
0.000 |
0.000 |
0.000 |
|
K |
1, 2, 4 |
1 |
1.3872 |
13.7671 |
1.37671 |
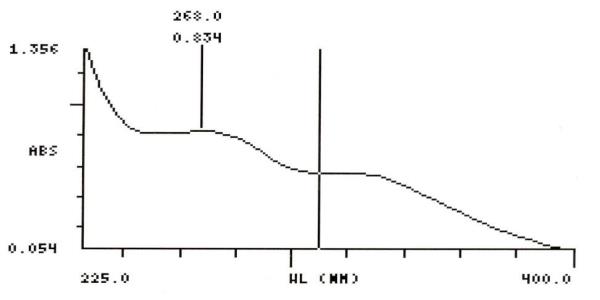
UV Spectral studies
|
Sample conc.(mg / ml) |
Solvent |
λ max (nm) |
λ min (nm) |
Abs. (λ max - λ min) |
|
0.625 |
Intestinal Fluid simulated without pancreatic pH=7.50.1 |
277 |
253 |
0.762- 0.630 |
|
0.9979 |
Gastric Fluid simulated without pepsin pH =1.20.1 |
268 |
250 |
0.834- 0.811
|
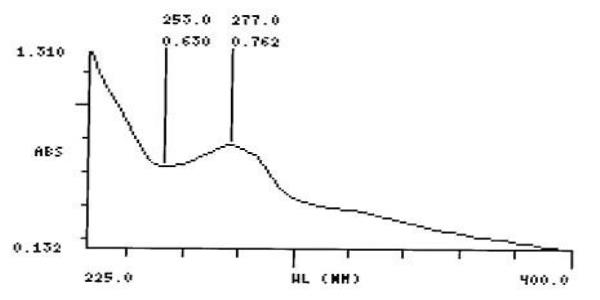
Gastric Fluid simulated without pepsin
Intestinal Fluid simulated without pancreatic
Chromatographic Studies
Thin layer chromatography (Wagner and Bladt, 1996)

A

B

C

D
TLC fingerprint of Pet. ether (60-80) °C extract (track 1) and MeOH extract (track 2)
|
Mobile phase Fig. |
A&C |
: |
Ethyl acetate, methanol, water (100:13.5:10) |
|
|
B&D |
: |
Toluene, ethyl acetate (93:7) |
|
Detection |
D |
: |
UV 366nm |
|
Derivatization |
A, B&C |
: |
Vanillin-Sulphuric acid-vis |
Pharmacological and toxicological studies
1.) Ethanolic extract)
The crude hexane extract demonstrated a potent antimicrobial effect against bacteria and a mild effect against fungi. Likewise, the hexane extract on cell cultures of the plant also showed mild bioefficacy against the selected microorganisms (Jain, 2003). The plant has been reported to have antimicrobial principles. Arnebins has associated for antimicrobial activity (Jain, 2000). The antimicrobial activities were tested against gram-positive and gram-negative bacteria and fungi.
Triterpenes of A. hispidissima was investigated and found to be active against selected bacteria and fungi. β-Amyrin demonstrated the maximum activity against E. coli (Jain, et al., 2003). The plant has been reported to contain shikonin derivatives, identified as arnebin-5, arnebin-6, teracrylshikonin, arnebinone and acetyl shikonin, which are known to have anti-inflammatory activity in carrageenan-induced paw edema and complete Freund's adjuvant-induced chronic arthritis in rats (Singh, 2000). Moreover, arnebin-1, significantly suppressed the development of chronic arthritis. Chemical investigations into the constituents of the roots of Arnebia hispidissima have yielded 3 new naphthaquinone which possess antibiotic and anti-cancerous properties. The quantification of naphthaquinones from in vivo and in vitro cell cultures of plant species and isolated compounds from intact plant tested for their swelling inhibitory potency. It has been reported that arnebin-1 was the major naphthaquinone both in vivo (0.62%) and in vitro (0.27%) cell cultures (Sing, 2004). The plant is reputed for the treatment of nervine pain and rheumatism. Other compounds, alkanninmonoacetate, alknannin α-dimethylacrylate. (±) – Alkamnin and three new naphthaquinones are reported as having antibiotic and anticancer activities (Hamdard, 1988).
The following pharmacological and safety evaluation studies were carried out (Derelanko 2002; Han, 2003) on the plant Arnebia hispidissima (70% ethanolic extract):
|
ACTIVITY |
RESULTS |
|||
|
Strong |
Moderate |
Mild |
Negative |
|
|
Analgesic |
|
✅ |
|
|
|
Antidepressant |
|
✅ |
|
|
|
Anticonvulsant |
|
|
✅ |
|
|
Adaptogenic (swim test) |
✅ |
|
|
|
|
Effect on rabbit jejunum |
|
✅ |
|
|
|
Effect on rat fundus |
|
|
✅ |
|
| Bronchiodilatory effect (G. pig isolated tracheal chain) | ✅ | |||
|
BP and heart rate |
|
|
|
✅ |
|
Effect on right rat atria |
|
✅ |
|
✅ |
| Erectile function (Rabbit corpus cavernous strip) | ||||
|
Studies on hematological parameters |
|
|
|
✅ |
|
Rectal temperature |
|
|
|
✅ |
|
Body weight |
|
|
|
✅ |
|
Vital organs |
|
|
|
✅ |
|
Mortality |
|
|
|
✅ |
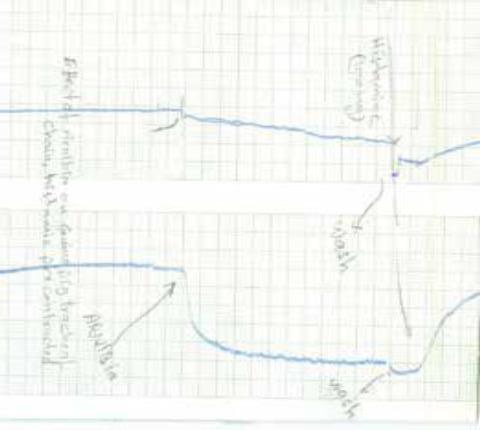
Bronchiodilatory effects of Arnebia on histamine
induced tracheal chain of the guinea pig
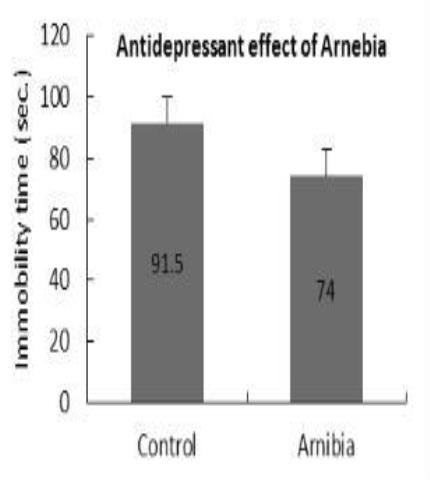
Antidepressant effect
Summary of the results
The plant extract administered for seven days demonstrated an increase in the hot plate latency time, showing significant analgesic activity using writhing test. It showed pronounced anti-depressant-like activity; and caused spasmodic action. It might produce relief of gastrointestinal spasm and improve the digestion; showed strong relaxant (bronchiodilatory) effects on histamine-induced tracheal chain of the Guinea pig. The plant could be used for bronchial asthma and allergic disorders. Adaptogenic activity test of the plant extract showed a significant increase in the swimming duration time, which indicates the anti-stress activity of the plant extract.
Antimicrobial activity
The aqueous extract of the whole plant was tested against Mycobacterium smegmatis, Candida tropicalis, Candida albicans, different strains of Methicillin-Resistant Staphylococcus aureus, different strains of ESBL-producing K. pneumonia, E. coli, Pseudomonas aeruginosa. The extract showed strong antibacterial activity against Methicillin Resistant Staphylococcus aureus and weak antimicrobial activity against Mycobacterium smegmatis, Candida tropicalis, and Candida albicans.
References
- B. Singh, M. K. Sharma, P. R. Meghwal, P. M. Sahu, and S. Singh. Anti-inflammatory activity of shikonin derivatives from Arnebia hispidissima. Phytomedicine 10: 375–380, 2003.
- El-Ghonemy, A. A. (1993). Encyclopedia of Medicinal plants of the United Emirates. 1st Edition. University of U.A.E.
- Fawzi, M. K. (1995). Weeds in the United Arab Emirates. University of U.A.E.
- Flora of Pakistan; www.efloras.org, p65.
- Hamdard ME, Bader Y, Khan MSY, and Shamsi MA, Jain SC, Singh B Jain R. Arnebins and antimicrobial activities of Arnebia hispidissima DC. Cell cultures. Phytomedicine, 1988, 6, 474-6.
- Hamid A. Khan, Indrani Chandrasekharan and A. Ghanim. Naphthazarins from Arnebia hispidissima. Phytochemistry. Volume 22, Issue 2, 1983, Pages 614-615
- Jain SC, Jain R, Singh B. Antimicrobial Principles from Arnebia hispidissima, Pharmaceutical Biology, 2003, 41: 231-233.
- Jain SC, Jain R, Singh. Antimicrobial principles from Arnebia hispidissima. Pharmaceutical biology, 2003, 4: 231 233.
- Jonbloed, M. V., Feulner, G. R., Boer, B. & Western, A. R. (2003). The comprehensive Guide to the Wild Flowers of the United Arab Emirates, Erwda, Abu Dhabi, U.A.E.
- Mandaville, J. P. (1990). Flora of Eastern Saudi Arabia. Kegan Paul International, Riyadh, Saudi Arabia.
- Miller, A. G. and M. Morris . 1988. Plants of Dhofar, The Southern Region of Oman. Traditional Economic and Medicinal Uses. Diwan of Royal Court Sultanate of Oman—Holmes MC Dougall, Edinburgh, Scotland.
- P.C.M. Jansen and D. Cardon. Plant Resources of Tropical Africa 3. Dyes and Tannins. PROTA foundation/Backhuys Publishers ( 2005) P.33
- Pakistan Journal of Botany, 42(2): 839-851, 2010
- Rastogi & Mehrotra, Compendium of Indian medicinal plants : vol. 4, PID, New Delhi,1995, p.67.
- S. C. Jain; R. Jain; B. Singh. Antimicrobial Principles from Arnebia hispidissima. Pharmaceutical Biology, Volume 41, Issue 4 June 2003 , pages 231 - 233
- Shahina AG. Handbook of Arabian medicinal plants. CRC Press, Inc. 2000 Corporate Blvd. N. W, Boca Raton. 1994.
- Singh B, Anti-inflammatory activity of shikonin derivatives from Arnebia hispidissima Phytomedicine, 2000 5: 375-380.
- Singh B, Sahu PM, Jain SC, Singh S Estimation of naphthaquinones from Arnebia hispidissima DC. In vivo and In vitro. I. Anti-inflammatory screening, Phytother Res, 2004, 18, 2: 154-9.
- V.P. Papageorgiou, A. N. Assimopoulou, E. A. Couladouros, D. Hepworth, and K. C. Nicolaou. The Chemistry and Biology of Alkannin, Shikonin, and Related Naphthazarin Natural Products.( REVIEWS). Angew. Chem. Int. Ed. 1999, 38, 270 -300.
- Western, A. R. (1986). The Flora of United Arab Emirates. An introduction. –Al Ain.
- Western, A. R. (1989). The Flora of United Arab Emirates. An introduction. Publications of the U.A.E. University.
