
Zingiber officinale / زنجبيل
Amomum zingiber L
Zingibil
Ginger
Zingibil
Zingiberaccae

Whole plant

Herbarium specimen
Ethnobotanical Characteristics
Description
Perennial herb up to 1m; rhizome fleshy. Up to 1.3 m. Leaves sessile, up to 15 x 2 (3) cm, linear-lanceolate, glabrous. Inflorescence on an up to 25 cm erect peduncle. Bracts green with a paler margin. Flowers yellow with a purple, yellow-spotted labellum; anther dark purple. (eFloras).
Habitat and Distribution
Cultivated throughout the tropical regions of Asia. Cultivated in some parts of Arabia.
Part(s) Used
Rhizome
Traditional and Medicinal Uses
The rhizome is used for bronchitis, as a carminative, for treating cough, cataracts and as a stimulant. The rhizome is cooked with salt and water and used as an expectorant. Extract of juice of rhizome is used as eye-drops. In Yemen, mixed with other plants used for constipation, as a purgative, against colds, catarrh and acidity of the stomach. Root ginger is widely used for culinary purposes and as a spice. The rhizomes (imported from India) are used with cinnamon and cloves and made into a tea for treating colds and as a general tonic. The drink is also used as an aphrodisiac Gazanfar, 1994). Ginger is useful in piles, rheumatism, headache, lumbago, pains, bleeding, chest congestion, cholera, cold, diarrhea, dropsy, nausea, stomachache, gastrointestinal disorders, vomiting and diarrhea. The fresh juice of ginger acts as a strong diuretic. The juice of the leaves is effective against helminthiasis and marasmus and related conditions of diarrhea and dysentery (Monograph of Unani Medicine, 2003). Ginger is extremely valuable in dyspepsia, flatulence, colic, vomiting, spas and other painful affections of the stomach, and the bowls unattended by fevers for cold, cough, asthma, dyspepsia and indigestion. Aromatic, carminative, stimulant to gastrointestinal tract, and stomachic; also digestive. Externally a local stimulant and rubefacient (Kapoor, 2001).
Pharmacognosy and Phytochemistry
Plant material Studied
Dried rhizome (Jamaican ginger)
General appearance
It is a pale yellowish branched rhizome and it is laterally compressed with longitudinally striated surfaces giving a coarse feeling when touched between fingers. When a branch is cut, it shows fibrous structures.
Microscopic characteristics
A transverse section of a branch of the rhizome is oblong in outline. As the sample is composed of an unpeeled rhizome, the outermost layers are composed of brown cork cells underlain by layers of collapsed parenchyma cells which are brownish-yellow in colour. These are surrounding the cortex which occupies a large zone and it consists of rounded and polygonal parenchyma cells and they are almost light yellow in colour. Many of the cortical parenchyma cells contain oblong or pear-shaped starch granules. Scattered between these cells are many yellow-coloured rounded or oval cells that contain droplets of yellow oil. The cortex is underlain by a clear endosperms layer with collapsed cells. The numerous vascular bundles which are light grey in colour are scattered in both cortex and the yellow-coloured stele. The xylem vessels are annularly, spirally or reticulately thickened. The vascular bundles are almost surrounded by a group of fibres which are long and they have thick walls which are pitted and one side of the walls is dentate. These fibres also have thin transverse septa which are observed at intervals. The central zone is occupied by the pith which consists of polygonal or rounded cells among which are scattered rounded or oval cells that contain droplets of a yellowish volatile oil.
Parts studied
Rhizome
A) TS of rhizome
B) Pith
C) Volatile oil droplets
Chemical constituents
The rhizome contains 1–4% essential oil and an oleoresin. the chief constituent is sesquiterpene .(-)-zingiberene, (+)-ar-curcumene, (-) – β-sesquiphellandrene, and β-bisabolene. Monoterpene aldehydes and alcohols are also present. Gingerols and shogaols (WHO, 1999).
gingerdione: 1-dehydrogingerdione (Reena , 2000).heptane, octane, isovaleraldehyde, nonanol, ethyl pinene,camphene, β-pinene, sabinene, myrecene, limonene, β-phellandrene and 1,8-cineole in essential oil. Gingediol,methylgingediol and their diacetates. Sesquiterpenes: sequithujene, cis-sesquisabinene hydrate and zingiberenol (2-methyl-6(trans-4´-methyl-4-hydroxycylohex-2-enyl)-hept-2-ene), car-3-ene,α-terpinene, terpineol,nerol,1,8cineole,zingiberene,neral,geranial,geraniol and geranyl acetate in essential oil from rhizomes.ar-Curcumen, α-farnesene, β-farnesene, linalool, β-sesquiphellandrene,gingerol, zingerone, shogaol, dihydrogingerol, hexahydrocurcumin in essential oil. (6)-dehydroginerdione,(10)-dehydrogingerdione,(6)-gingerdione and (10)-gingerdione and (6)-gingerol from roots. Gingerdiones, hexahydrocurcumin and desmethylhexahydrocurcumin.Gingerols I,II and II from rhizomes.Asparticacid,threonine, serine, glycine, cysteine, valine, isoleucine, leucine and arginine from aerial parts and tuber. β-Bisabolene, ar-curcumen,α-farnesene, zingiberene, camphene, 1,8-cineole, geranial, neral and phellandrene in oil from rhizomes.
(6)-shogaol,(6)-dehydrogingerdione,(8)-and (10)-gingerols are active constituents. Zingiberene, α-curcumene, α-copaene, undecan-2-one, neral and geranial from root essential oil.Zerumbone, zerumbodienone, humulene epoxide I and humulene epoxide II from roots.galanolactone and (E)8,17-epoxylabd-12-en-15,16-dial along with (6)-shogaol,(6)-,(8)- and (10)-gingerols from roots. Diarylheptenones: gingerenone A, B, and C and isogingerenone B from rhizomes (Rastogi, 1991, 1993, 1995, 1998).
The following chemical studies have been carried out (Quality Control methods, 1998; Evans, 1996) on the rhizome of Zingiber officinale (ZCHRTM unpublished work):
Physicochemical constants
Loss of weight in drying at 105°C : 10.90
Absolute alcohol solubility : 4.00
Water solubility : 16.00
Successive extractives (%)
Petroleum ether (60-80)°C : 3.05
Chloroform : 1.05
Absolute alcohol : 1.90
Ash values (%)
Total ash : 8.50
Water soluble ash : 1.30
Acid insoluble ash (10% HCl) : 0.20
pH values (aqueous solution)
pH of 1% solution : 4.58-4.62
pH of 10% solution : 4.31-4.32
Elemental analyses
Ash values (British Herbal Pharmacopeia)
Assay and identification of element (AOAC International)
|
Apparatus |
AA-6800 Shimadzu-Flame method |
||||
|
Element |
Std. conc. µg/ml (ppm) |
Sampleconc.mg/ml |
Sample absorbance |
Actual conc. mg/ml |
Actual conc. (%) |
|
Cr |
1, 2, 4 |
9.995 |
0.0000 |
<0.0001 |
<0.001 |
|
Zn |
0.25, 0.5, 1 |
9.995 |
0.1388 |
0.04169 |
0.004169 |
|
Cu |
1, 2, 4 |
9.995 |
0.006 |
0.00722 |
0.00072 |
|
Fe |
1, 2, 4 |
0.9086 |
0.0274 |
0.05051 |
0.005051 |
|
K |
1, 2, 4 |
0.9086 |
1.4268 |
10.88296 |
1.088296 |
|
Pb |
1, 2, 4 |
9.995 |
0.0000 |
<0.0001 |
<0.00001 |
|
Cd |
0.125,0.25,0.5 |
9.995 |
0.0000 |
<0.00001 |
<0.000001 |
|
Ca |
5, 10, 20 |
0.826 |
0.0193 |
5.529864 |
0.552986 |
|
Mg |
0.25, 0.5, 1 |
0.0826 |
0.2825 |
2.90884 |
0.290884 |
|
Na |
1,2,4 |
0.9086 |
0.09992 |
2.2693 |
0.22693 |
| 1ppm conc. = 1µg/ml; Actual conc. (%) =Actual conc.(ppm)x0.0001 [1ppm=0.0001%] | |||||
UV Spectral studies
|
Ultraviolet Spectrum (USP reference) |
||||
|
Apparatus |
Beckman DU 520 general purpose UV/VIS spectrophotometer. |
|||
|
Sample conc. (mg / ml) |
Solvent |
λ max (nm) |
λ min (nm) |
Abs. (λ max - λ min) |
|
0.60636 |
Intestinal Fluid simulated without pancreatic pH=7.50.1 |
205.5 277.5 |
260.5 |
2.007 0.323-0.296 |
|
0.83666 |
Gastric Fluid simulated without pepsin pH =1.20.1 |
204 280.5 |
257.5 |
1.857 0.324- 0.276 |
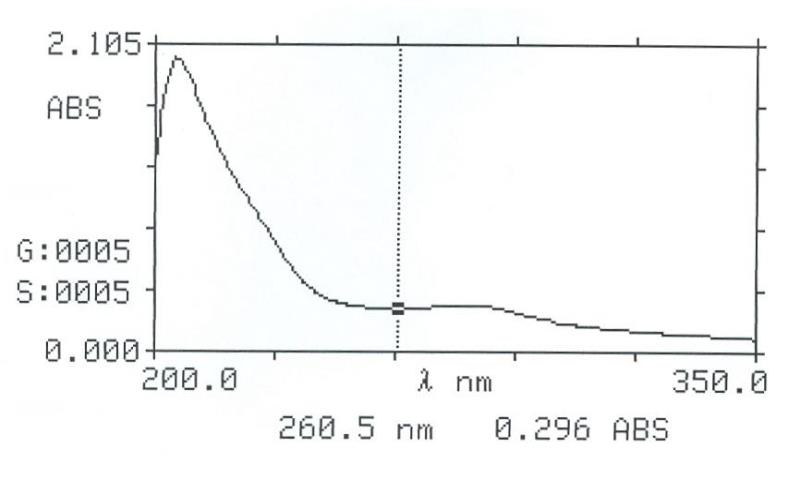
Intestinal Fluid simulated without pancreatic
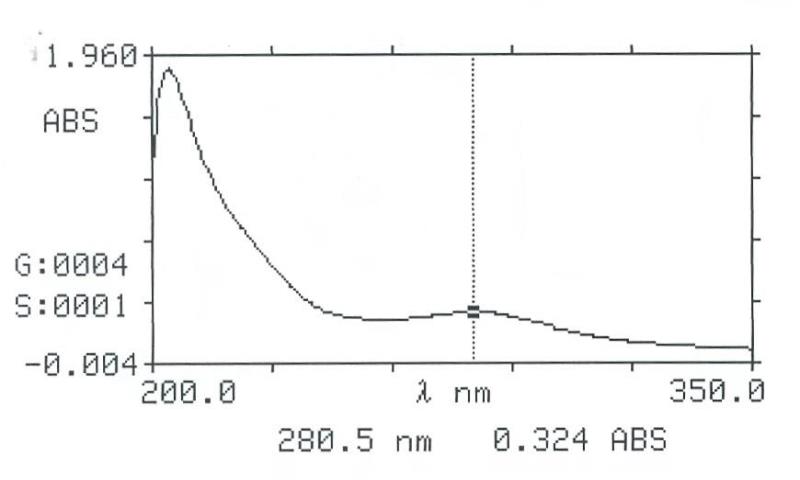
Gastric Fluid simulated without pepsin
Chromatographical Studies
Thin layer chromatography (TLC): Wagner and Bladt, 1996

A

B

C

D
TLC fingerprint of Petroleum ether 60-80°C (track 1) and MeOH extract (track 2)
|
Mobile phase Fig. |
A&D |
: |
Toluene, ethyl acetate (93:7) |
|
|
B&C |
: |
Toluene, ethyl formate, formic acid (5:4:1) |
|
Detection |
A&B |
: |
UV 254nm |
|
Derivatization |
C&D |
: |
Vanillin-Sulphuric acid under normal light |
Pharmacological and Toxicological Studies
Literature and reported information of the plant: General pharmacological studies were performed on gingerol and shogaol which are the pungent constituents of ginger (Zingiber officinale Roscoe). Intravenous (IV) administration of gingerol at (1.75-3.5 mg/kg) or shogaol (at 1.75-3.5 mg/kg), and oral administration of both (at 70-140 mg/kg) resulted in the inhibition of spontaneous motor activity, antipyretic and analgesic effects and prolonged hexobarbital-induced sleeping time. These effects of shogaol were mostly more intensive than that of gingerol. Shogaol showed an intense antitussive effect in comparison with dihydrocodeine phosphate. In the electro-encephalogram of cortex, the low amplitude fast wave pattern was observed for 5 min after IV administration of shogaol. It then changed to the drowsy pattern, which was restored after 60 min. In the gastrointestinal system, shogaol intensively inhibited the traverse of charcoal meal through the intestine in contrast with gingerol after IV administration of 3.5 mg/kg. However, shogaol facilitated such an intestinal function after oral administration of 35 mg/kg. Both shogaol and gingerol suppressed gastric contraction in situ, and the suppression by the former was more intensive than that by the latter. In the cardiovascular system, both shogaol and gingerol produced depressor response at lower doses on the blood pressure. At high doses, both drugs produced three phase pattern (Suekawa, 1984). An ethanolic decoction of Zingiber officinale (200 mg/kg) fed orally for 20 days produced a significant antihyperglycemic effect (P<0.01) in diabetic rats (Bhandari, 1998). Studies on the effects of ginger in albino rats showed cytoprotective and anti-ulcerogenic effects of ginger (Al-Yahiya, 1989).
Ginger is considered to act directly on the human gastrointestinal system to reduce nausea (Holtmann, 1989). Ginger has been shown to reduce the symptoms of motion sickness associated with travel by boat and, to a lesser extent, in vehicles up roads on mountains (Grontved, 1988; Ribenfeld, 1999; Careddu 1999). Two double-blind clinical trials showed that ginger might reduce nausea due to anesthesia following surgery (Bone, 1990; Phillips, 1993). However, one trial did not confirm this benefit (Arfeen, 1995). A preliminary trial has suggested that ginger may help to prevent chemotherapy-induced nausea (Meyer, 1995). While ginger is a popular remedy for nausea of pregnancy, it has only been clinically studied for very severe nausea and vomiting, known as hyperemesis gravidarum (Langner, 1998). Test tube studies showed that ginger contains some compounds that cause a chromosomal mutation that could be life threatening. However, the current clinical research, combined with the fact that ginger is widely used in the diet of certain cultures, suggests that prudent use of ginger at the rate of up to 1.0 g/day is safe for morning sickness.
Ginger is considered as tonic for the digestive system as it stimulates digestion and tones the intestinal muscles (Bradley,1992). Ginger may protect the stomach from the damaging effect of alcohol and non-steroidal anti-inflammatory drugs (NSAIDs, such as ibuprofen) and may help prevent ulcers (Al-Yahya ,1989).
Ginger also supports cardiovascular health. Ginger may make blood platelets less sticky and less likely to aggregate (Bordia, 1997; Verma, 1993). However, not all human research has confirmed this (Lumb, 1994; Janssen, 1996).
The efficacy of ginger in the prevention of postoperative nausea and vomiting was studied in a double-blind, randomized, controlled trial in 108 ASA 1 or 2 patients undergoing gynecological laparoscopic surgery under general anesthesia. These studies concluded that ginger BP in doses of 0.5 or 1.0 gram is ineffective in reducing the incidence of postoperative nausea and vomiting (Arfeen, 1995).
The effectiveness of ginger (Zingiber officinale) as an antiemetic agent was compared with placebo and metoclopramide in 60 women who had major gynecological surgery in a double-blind, randomized study. The administration of antiemetic after operation was significantly greater in the placebo group compared to the other two groups (p < 0.05) (Bone, 1990).
In a placebo-controlled study, the effect of ginger and fenugreek was examined on blood lipids, blood sugar, platelet aggregation, fibrinogen and fibrinolytic activity. Ginger did not affect the blood lipids and blood sugar (Bordia, 1997). Ginger on thromboxane synthetase activity was found dose-dependent, or only occurs with fresh ginger. The study showed that 2 g of dried ginger is unlikely to cause platelet dysfunction when used therapeutically (Lumb, 1994).
In a double-blind randomized placebo trial, the effect of the powdered rhizome of ginger was tested on sea sickness. Ginger root reduced the tendency of vomiting and cold sweating significantly than the placebo did (p < 0.05) (Grontved, 1988). The effect of powdered ginger root upon vertigo and nystagmus following caloric stimulation of the vestibular system was studied in eight healthy volunteers in a double-blind crossover placebo trial (Grontved, 1986). The effect of ginger root (Zingiber isrhizoma) on gastrointestinal motility was examined based on its ability to enhance charcoal meal transport in mice. The plant and its active constituents have gastrointestinal motility enhancing effect (Yamahara, 1990).
The effects of gingerol and shogaol were similar to or slightly weaker than those of metoclopramide and donperidone. Dietary supplementation ginger in 20 healthy male volunteers for 7 days was found to enhance platelet aggregation to a significant extent (Verma, 1993).
The following pharmacological and safety evaluation studies (Derelanko 2002; Han, 2003). were carried out on the plant decoction of Zingiber officinalis.
|
ACTIVITY |
RESULTS |
|||
|
Strong |
Moderate |
Mild |
Negative |
|
|
Analgesic (Hot plate) |
|
|
|
√ |
|
Anti-diabetic activity |
|
√ |
|
|
|
Antidepressant (TST) |
|
|
|
√ |
|
Anti-stress activity (Swimming test) |
√ |
|
|
|
|
Anti-gastric ulcer effect (NaOH rat model) |
√ |
|
|
|
|
Antithrombotic effect |
|
√ |
|
|
|
Effect on guinea pig tracheal chain |
|
|
|
√ |
|
Effect on rabbit jejunum |
|
√ |
|
|
|
Effect on guinea pig ileum |
|
|
√ |
|
|
Effect on rat fundus |
|
√ |
|
|
|
Effect on detrusor muscle |
|
√ |
|
|
|
Effect on right rat atria (HR) ↓ |
|
|
√ |
|
|
Acute toxicity on mice |
|
|
|
√ |
|
Locomotor activity test |
|
|
√ |
|
|
Motor co-ordination (grip strength & motor activity |
|
|
|
√ |
|
Rectal temperature |
|
|
|
√ |
|
Body weight |
|
|
|
√ |
|
Mortality |
|
|
|
√ |
|
LD50 |
> 10 g/kg p.o. in mice |
|||
Summary of the Results
The plant decoction showed moderate anti-diabetic activity, the combination of Zingiber and Musa exhibited anti-gastric ulcer effects in rats, and a moderate contraction effect on acetylcholine treated detrusor muscle. The decoction was evaluated for its safety in mice following acute toxicity tests. Acute administration of the plant decoction did not produce any noticeable toxic effects in the mice at the doses tested.
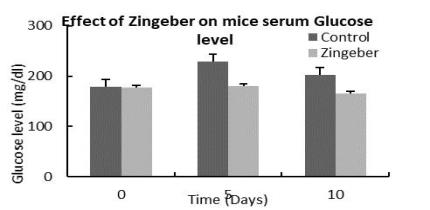
Effect on serum Glucose level
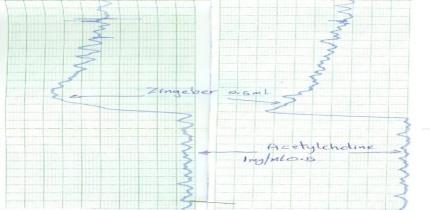
Effect on detrusor muscle
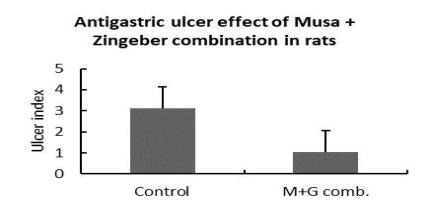
Antigastric ulcer effect
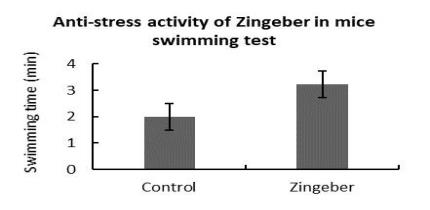
Anti-stress activity
Antimicrobial activity
The aqueous extract of rhizomes did not inhibit the growth of Mycobacterium smegmatis, C. tropicalis, different strains of Staphylococcus aureus (Including ATCC 257) as well as strains of Methicillin Resistant Staphylococcus aureus, strains of E. coli (Including ATCC UN 109), different strains of ESBL-producing K. pneumonia, E. coli, Pseudomonas aeruginosa.
References
- Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS, Tariq M. (1989).Gastro protective activity of ginger in albino rats. Am J Chinese Med, 17:51-56.
- Arfeen Z, Owen H, Plummer JL, Ilsley AH, Sorby-Adams RA, Doecke CJ. (1995). A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting.Anaesth Intensive Care. 23:449-452.
- Bauer AW, Kirby WMM, Sheriss JC, Turck M. (1966) Antibiotic susceptibility testing by standardized single method. Am J Clin Pathol; 45:493–6.
- Bhandari Uma, Kanojia Raman, Pillai K. K, (1998). Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats .Blumenthal M, Busse WR, Goldberg A, et al. (eds). The Complete Commission E Monographs: Therapeutic Guide to Herbal Medicines. Boston, MA: Integrative Medicine Communications, 135-136.
- Bone ME, Wilkinson DJ, Young JR, et al. (1990). Ginger root: A new antiemetic: The effect of ginger root on postoperative nausea and vomiting after major gynaecological surgery. Anaesthesia, 45:669–671.
- Bordia A, Verma SK, Srivastava KC. (1997).Effect of ginger (Zingiber officinaleRosc) and fenugreek (Trigonella foenumgraecum L) on blood lipids, blood sugar, and platelet aggregation in patients with coronary artery disease.Prostagland Leukotrienes Essential Fatty Acids. 56:379-384.
- Bradley PR (ed). (1992). British Herbal Compendium,vol 1. Bournemouth, Dorset, UK: British Herbal Medicine Association, 112-114.
- British Herbal Pharmacopoeia (1996).4th Ed.: British Herbal Medicine Association (BHMA).
- Brown DJ. Herbal Prescriptions for Better Health. Rocklin, CA: Prima Publishing, 1996, 111–118.
- Careddu P. (1999). Motion sickness in children: Results of a double-blind study with ginger and dimenhydrinate. Healthnotes Rev Complementary Integrative Med, 6:102-107.
- Derelanko, M. J., & Hollinger, M. A. (2002). Hand book of toxicology. (2nd ed.).Boca Raton, USA: CRC Press.
- Evans, W.C (1996).Trease and Evans’ Pharmacognosy,(14th ed,p.105 )Saunders ,London.
- Flora of Pakistan.e-floras, Web.
- Ghazanfar, S. A. (1994).Handbook of Arabian Medicinal Plants. Boca Raton, USA: CRC Press.
- Grontved A, Brask T, Kambskard J, Hentzer E. (1988). Ginger root against seasickness. A controlled trial on the open sea.ActaOtolaryngol (Stockh). 105:45-49.
- Grontved A, Hentzer E. (1986). Vertigo-reducing effect of ginger root.A controlled clinical study.ORL J OtorhinolaryngolRelat Spec. 48:282-286.
- Han, J., & Hoosier, G. L. V. J. (2003). Hand book of laboratory science, animal models. (Second ed., Vol. II). USA: CRC Press.
- Holtmann S, Clarke AH, Scherer H, Hohn M. (1989).The anti-motion sickness mechanism of ginger.A comparative study with placebo and dimenhydrinate.ActaOtolaryngol (Stockh), 108:168–174.
- Janssen PL, Meyboom S, van Staveren WA, de Vegt F, Katan MB. (1996). Consumption of ginger (Zingiberofficinale Roscoe) does not affect ex vivo platelet thromboxane production in humans.Eur J ClinNutr, 50:772-774.
- KapoorL.D.(2001). Handbook of Ayurvedic medicinal plants, CRC Press, p.341
- Langner E, Greifenberg S, Gruenwald J. (1998). Ginger: History and use. AdvTher, 15::25–44 [review].
- Lumb AB. (1994). Effect of dried Ginger on human platelet function.ThrombHemostas, 71:110-111.
- Meyer K, Schwartz J, Craer D, Keyes B. (1995). Zingiber officinale (ginger) used to prevent 8-Mop associated nausea. Dermatol Nursing, 7:242–244.
- Monograph of Unani Medicine Vol.1.Drugs Cont. &Trad.Med.Div. Islamabad, Pakistan, p.570, 2003.
- Mothana R A A, Abdo SA ,Hasson S, Althawab FM, Alaghbari SA, Lindquist U.( 2008)
- Antimicrobial, antioxidant and cytotoxic activity and phytochemical screening of some Yemeni medicinal plants. Evid Based Complment Alternat Med. 7(3):323-30.
- Official Methods of Analysis of AOAC International (1999).16th. Ed.Vol.I and II
- Phillips S, Ruggier R, Hutchingson SE. (1993). Zingiber officinale (ginger)-an antiemetic for day case surgery.Anaesthesia, 48:715–717.
- Quality control methods for medicinal plant materials (1998).World Health Organization, Geneva.
- Rastogi., &Mehrotra, (1991).Compendium of Indian medicinal plants.(Vol. 2, p718). New Delhi: PID
- Rastogi., &Mehrotra, (1993).Compendium of Indian medicinal plants.(Vol. 3, p. 619). New Delhi: PID
- Rastogi., &Mehrotra, (1995).Compendium of Indian medicinal plants.(Vol. 4, p. 774). New Delhi: PID
- Rastogi., &Mehrotra, (1998).Compendium of Indian medicinal plants.(Vol. 5, p. 908). New Delhi: PID
- Reena Charles, S. N., Garg, U., &kumar, S. (2000).New gingerdione from the rhizomes of zingiber officinale.Fitoterapia, (71), 716-718.
- Ribenfeld D, Borzone L. (1999). Randomized double-blind study comparing ginger (Zintona) with dimenhydrinate in motion sickness. Healthnotes Rev Complementary Integrative Med 6:98-101.
- Suekawa M, Ishige A, Yuasa K, Sudo K, Aburada M, Hosoya E. (1984). Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn. 7:836-848.
- Verma SK, Singh J, Khamesra R, Bordia A. (1993). Effect of ginger on platelet aggregation in man. Indian J Med Res, 98:240-242.
- Wagner, H. Bladt, S.(1996). Plant Drug Analysis-A Thin layer Chromatography Atlas, (2nd Ed.) Springer-Verlag, Berlin Heidelberg.
- WHO monographs on selected medicinal plants, (1999). WHO monographs on selected medicinal plants. (Vol. 1, p. 280).World Health Organization: Geneva.
- Yamahara J, Huang QR, Li YH, Xu L, Fujimura H. (1990). Gastrointestinal motility enhancing effect of ginger and its active constituents.Chem Pharm Bull, 38:430-431.
- ZCHRTM unpublished work
