
Lawsania inermis / حنة
Lawsania alba Lam.
Henna,Alcanna, Mignontte tree,Egyptian Privet
Henna
Henna
Lythraceae

Flowers
Herbarium Sample

Fruits
Ethnobotanical Characteristics
Description
Glabrous much – branched deciduous shrub up to 3-5(7) m high; older branches often spinescent. Leaves simple, opposite, elliptic lanceolate, sessile or with very short petiole, entire margin, 1.5-3.5 cm long & 0.5-1.3cm broad. Inflorescence paniculate crymbs at end of branches; flowers whitish pink, c.10cm across fragrant with leafy bracts, 4-merous,petals greenish yellow 0.5cm long, stamens 4-8. Fruit capsule, globose, 0.5-0.7cm in diameter, with a persistent base of style. Seeds numerous, smooth, pyramidal, orange brown.
Habitat & Distribution
Indigenous to Arabia, Iran , Asia, North Africa and Egypt; found wild on dry hillsides, rarely in dry deciduous forest. Now cultivated in many countries: Morocco, China, USA and Australia. In UAE naturalized through cultivation.
Part(s) used
leaves, flowers, seeds, roots.
Properties & Medical Uses
Derived from the Arabic name Alkenna, henna is used for dying hair and body. The prophet Mohammed ( P. B. U. H) is said to have used it to redden his beard; and still commonly used all over the world. The plant is astringent, cooling, Leaves antifungal, antimicrobial, emmenagogue; used for cough, asthma, and skin diseases, burns; blood circulation, hypertension, cardiac problems, external wound, bruises, varicose veins and to produce abortion. Leaf juice used for spermatorrhea; dye for hair and heat, for leprosy in Africa folk. Flowers used in perfumery. Volatile oil used externally to cool the body.
Pharmacognosy and Phytochemistry
Parts studied
Leaves
Microscopical Description
It has finely branched pinnate venation. The lamina is isobilateral and both epidermises are similar in appearance and are composed of polygonal cells with thin, straight or slightly sinuous walls. The anomocytic stomata are numerous and are fairly scattered on both surfaces. Cells of the lower epidermis over the veins are more elongated and have a striated cuticle. The epidermal cells of the petiole are similar to those of the lamina but their walls are slightly beaded and the cuticle is strongly striated but stomata are absent. The transverse section of the lamina shows two layered palisade tissues under both epidermises with the palisade cells under the lower epidermis slightly shorter than those under the upper one. The spongy mesophyll cells contain cluster crystals of calcium oxalate which are normally large and sometimes show a dense brown centre. The pericylce of the midrib and larger veins contain small groups of lignified fibres which have moderately thick walls with few pits. The powder is yellowish green in colour with a slight odour and a faintly bitter taste. It shows fragments of all the above-mentioned characteristic features( DPS ZCHRTM unpublished results).
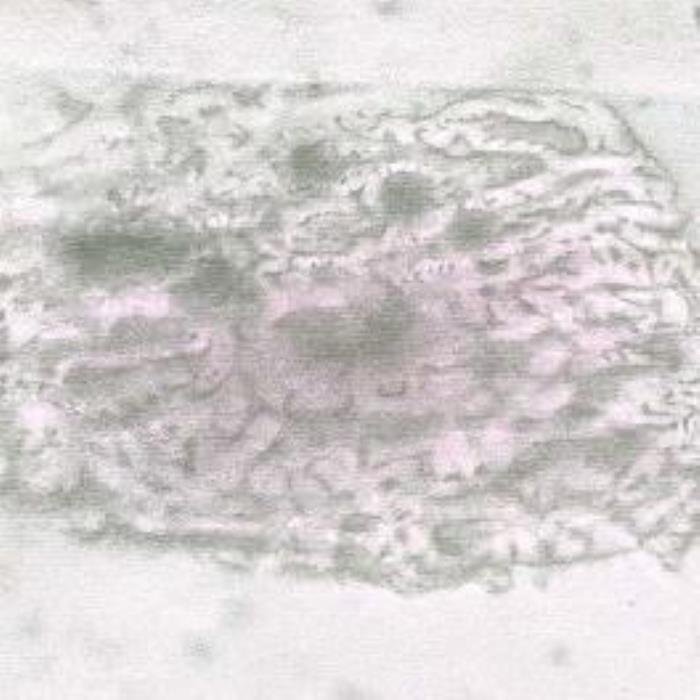
a) Leaf fragment
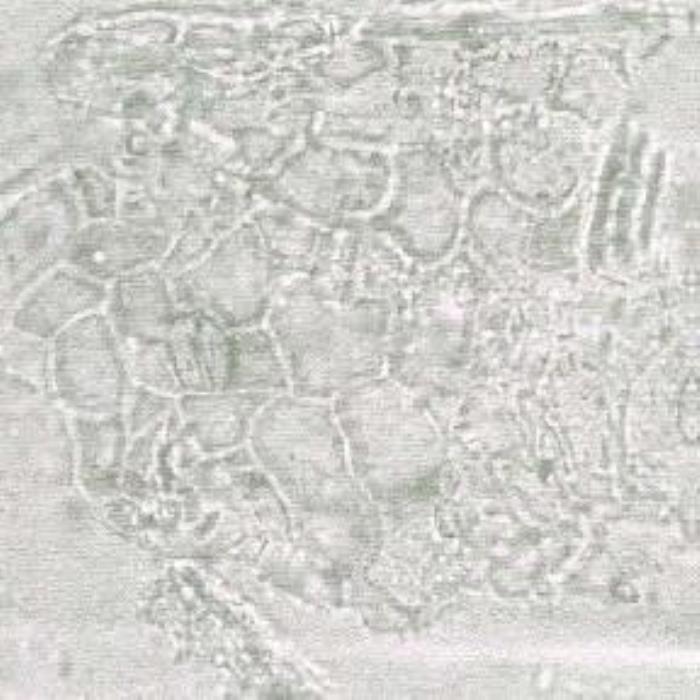
b) Lower epidermis
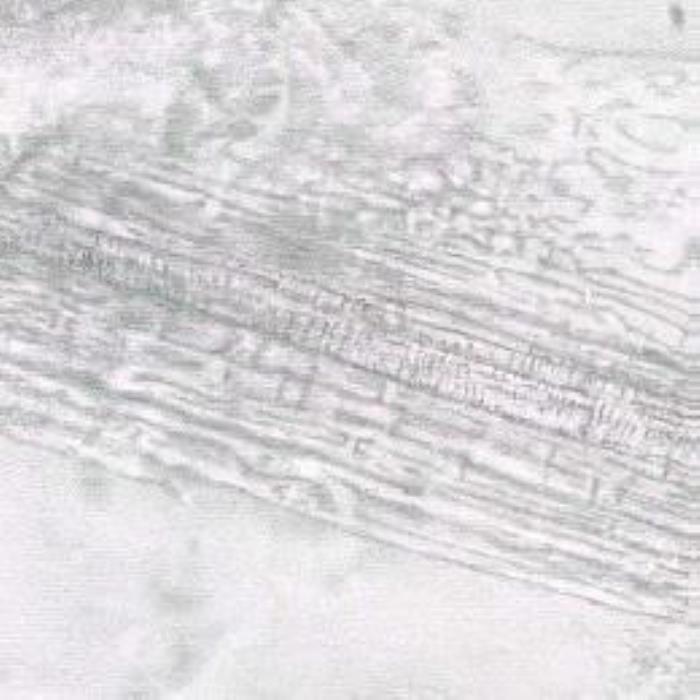
c) Petiole xylem vessels
- (a). Leaf fragment showing a part of the mesophyll cells containing pigments and cluster crystals of calcium oxalate with dark centers.
- (b). Surface view of the lower epidermis of the leaf lamina showing somewhat shining beady cell walls besides some stomata.
- (c). Petiole xylem vessels at the idle surrounded by layers of parenchyma (narrow, longitudinal) and xylem fibers, which are also encircled by phloem sieve tubes, parenchyma and thick fibers. (Magnifications: All x 400).
Organoleptic characteristics
Appearance: Solid powder
Colour: Brown
Odour: Pleasant
Taste: Bitter
Physicochemical constants
Loss in weight on drying at 105°C (%): 8.45-8.80
Solubilities (%)
Alcohol solubility: 11.2
Water solubility: 16.80
10% ethanolic extractive: 45.60
Ash values (%)
Total ash: 10.40
Water soluble ash: 0.80-1.00
Acid-insoluble ash: 3.80-4.00
Successive extractive (%)
Petroleum ether (60-80°c): 7.50-7.60
Chloroform: 2.00
Absolute alcohol: 25.10-31.80
Distilled water: 12.00-12.90
pH values
pH of 1% solution: 4.19
pH of 10% solution: 3.98
The above results are under process of publication( DPS ZCHRTM unpub results).
Chemical constituents
Naphthalene derivatives (1,4-naphthaquinone) in particular lawsone (2-hydroxy, 4 -naph thaquinone) C10H6O3, M.P.190 arising during dehydration of the leaves out of the precursor 1,2,4 trihydroxy naphthalene-4-beta-D-glucoside Tannins. (DPS, ZCHRTM unpub results; PDR 1998; Kamil et.al 2002).
Pharmacological and Toxicological studies
Lawsonia showed significant inhibition of tumor burden in two tumor model systems studied (Dasgupta et .al., 2003).
People of different age and hair length were treated with the mixture of the plants (Lawsonia; Trigonella; Hibiscus; and Artemisia ) which was found effective against the head lice (El-Basheir et. al., 2003). The pharmacological and toxicological studies carried out in our laboratory and the results in brief, on Lawsonia inermis (10% ethanolic extract) have been given below. The results presented without references showed unpublished data (unpublished, ZCHRTM, DBMS):
|
ACTIVITY |
RESULTS |
|
Effect on GIT smooth Muscle- Isolated rabbit jejunum |
Produced significant reduction in amplitude. |
|
Effect on GIT smooth Muscle-Isolated rat fundus |
Produced significant contraction. |
|
Hepatoprotective activity/ Hepatotoxicity activity |
Results from these studies are not conclusive. |
|
Hair growth study-Weight change study |
Hair growth increased significantly in rats. |
|
Hair growth study-Thallium chloride method |
Thallium chloride-induced Alopecia produced. |
|
Gross behavioral studies- Tremor/Twitches |
No tremors observed. |
|
Gross behavioral studies-Writhing |
No Writings observed. |
|
Gross behavioral studies-Diarrhea, Urination |
No diarrhea observed. |
|
Mortality |
No death recorded. |
|
Motor co-ordination (String &Platform test) |
Motor co-ordination not affected. |
|
Acute toxicity studies- |
No toxic symptoms observed. |
|
LD50 evaluation |
>10 g/kg. |
Summary of the results
It was found that the plant produced significant hepatoprotective activity in carbon tetra chloride- induced model; the plant extract also showed marked reduction in the amplitude of spontaneous contraction of isolated rabbit jejunum which could be utilized to produce laxative activity; Lawsonia extract applied topically enhanced hair growth in shaven rats. The plant extract produced contraction of isolated rat fundus strip; No obvious toxic symptom were noticed.
Antimicrobial activity
In vivo studies on guinea pigs and mice showed that the herb at a dose of 5 mg/kg body weight led to significant resolution of experimental tuberculosis following infection with M. tuberculosis. Tuberculostatic activity of henna (Sharma , 1990). The Lawsonia bark extract was found to possess a broad fungitoxic spectrum and non phytotoxicity spectrum with fungistatic inhibitory at 1:10 (W/V). Further the fungitoxicity of the extract remained unaltered at high temperature, on autoclaving and after long storage. On chemical investigation, the antifungal factor was found to be 2-hydroxy-1,4-naphthoquinone (Lawsone). The minimum effective dose against test organism was found to be 1000 ppm. (Singh et al., 1989; Tripathi et al., 1978; Galal et al., 1965 ) . On the other hands 2-hydroxy-1,4-naphthoquinone reported as a weak bacterial mutagen for Salmonella typhimurium strain TA98 or was more clearly mutagenic for strain TA 2637 (Kirkland et al. 2003).
References
- Andrews, F.W. (1952) The Flowering Plants of Anglo-Egyptian Sudan; Vol I,. Arbroath, Scotland.
- Dasgupta T, Rao AR, Yadava PK. (2003) Modulatory effect of henna leaf (Lawsonia inermis) on drug metabolizing fazes I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Mol Cell Biochem. 245(1-2): 11-22.
- Department of Biomedical Sciences, Zayed Complex for Herbal Research and Traditional Medicine, Unpublished results.
- Department of Pharmacognostic Sciences, Zayed Complex for Herbal Research and Traditional Medicine (ZCHRTM),unpublished results.
- El-Basheir ZM, Fouad MA. (2003) A preliminary pilot survey on head lice, pediculosis in Sharkia Governorate and treatment of lice with natural plant extracts. J Egypt Soc Parasitol. 32(3): 725-36.
- Galal EE, Sabbagh H, Ghali B, Fawazi G. Screening of antifungal activity of some Egyptian plant extracts; "Lawsonia inermis". J Egypt Med Assoc. 1965; 48:Suppl:153-60.).
- Kamil M, Sheikh El O. M, , Faizan A, Gunasekhar C, Mazen A Naji. Henna (Lawsonia inermis) – A Paharmacognostic and Phytochemical Study; The 2nd Arab Conference on Medical Plants. 13-15 May 2002. Arabian Gulf University, Bahrain.
- Kirkland D, Marzin D. (2003) An assessment of the genotoxicity of 2-hydroxy-1, 4-naphthoquinone, the natural dye ingredient of Henna. Mutat Res. 6; 537(2): 183-99.
- Kotb, T. F. Medicinal Plants in Libya.(1985) Arab Encyclopedia House. Tripoli-Libya.
- Mandaville, J. P. Flora of Eastern Saudi Arabia. (1990) Kegan Paul International Ltd. England.
- Miller A.G., Morris M. Plants of Dhofar, The southern Region of Oman: Traditional, Economic and Medicinal Uses.(1987) Office of the Advisor for conservation of the Environment, Sultanate of Oman.
- PDR for Herbal Medicines, Medicinal Economics Company, Montvale, New Jersey, P. 930,1998.
- Singh VK, Pandey DK. Fungitoxic studies on bark extract of Lawsonia inermis against ringworm fungi. Hindustan Antibiot Bull. 1989 31(1-2): 32-5.
- Sharma, V.K ( 1990), Tuberculostatic activity of Lawsonia inermis Linn. Tubercle. 1990 71(4): 293-5.
- Tripathi RD, Srivastava HS, Dixit SN. A fungitoxic principle from the leaves of lawsonia inermis lam. Experientia. 1978 34(1): 51-2.
- Warrier, P.K., NambiarV. P.K & Ramankutty, C( edit.)( 1995) Indian Medicinal Plants . (1962) Vol. I, Orient Longman, Kottakal, India.
- مختار سالم. أعشاب لكنھا دواء. ( 1987 ) دار المریخ للنشر، الریاض- المملكة العربیة السعودیة
