
Ziziphus spina- Christi / السدر
Rhamnus spina-christi L., Ziziphus africana Mill.
Sidr, Nabaj (fruit), Nibs, Jabat, Zejzaj, Zefzoof, Ardeg, Ghosl,Kanar
Christ`s thorn
Sidr, Nebeq
Rhamnaceae

Herbarium Sample

Branches

Fruits
Ethnobotanical Characteristics
Description
Armed shrub or tree to 10 m tall; branches whitish brown, often zig-zagging, thinly hairy when young; stipules paired, spibous, one of each pair straight to 2.5 cm long the other shorter & hooked (up to 0.5cm long). Leaves alternate, 3-nerved from the base, ovate to elliptic, 2-4 (-6) cm long x 1.5-3 cm across, tip rounded or obtuse, margin minutely crenate-serrate, base round to sub cordate, glabrous or thinly haring beneath along the veins. Flowers in dense dusters in the axils of the leaves. Calyx x 5 – lobed, cup-shaped at base; lobes ovate – triangular, c.2 x 1.5 mm, densely pubescent, deciduous – petals yellowish, 5, free, obovate and shortly clawed, c.1.5 x 1 mm, hooded at apex. Stamens 5, opposite the petal, inserted at the base of the flat and 10- lobed disc. O vary 2-locular, loculi 1-ovulate, styles short 8 divided above into two lobes. Fruit a drupe, globose, c.1cm in diameter, glabrous, yellowish or reddish brown, edible; seeds ovate, 6-7 x 5-6 mm brown.
Habitat & Distribution
Widespread in tropical and subtropical regions. The genus Z. spina-christi is widely distributed because it is cultivated & has been naturalized across a large area from N. Africa east to Afghanistan & NE India. In UAE found in wadis, different landscape, and it is widely cultivated all over the UAE. The plant is mentioned in holy Quran and very much used in Arab countries.
Part(s) Used
Fruits, bark, seeds and leaves
Traditional & Medicinal Uses
In UAE : the fruits edible and nutritive, leaves used for chest and stomach problems, bone fractures, bleeding of uterus and for hair and skin care. In Iberis P the plant claimed to be a blood purifier, good for treatment of liver, epilepsy, dermatitis and chest problems. In different folk medicine the plant is used for scorpion stings, cough, constipation, pneumonia, headache, dysentery, intestinal worms, fever, eye diseases and inflammations of throat and pharynx.
Pharmacognosy and Phytochemistry
Parts studied
Leaves
Microscopical Description
Transverse section of the leaf shows an isobilateral structure where the palisade tissue is found beneath both epidermises. The cuticle is thin and it is faintly and irregularly striated. The upper epidermis consists of large anticlinal oval to oblong cells though they look polygonal from a surface view. They have thin straight cell walls and some of them contain single emerald green crystals. The mesophyll palisade tissue consists of two layers or rod-like elongated cells with straight cell walls that are loosely held together. The cells of the palisade tissues are very rich in coloured crystalline substances; some cells contain cluster crystals of calcium oxalate or mixtures of different crystalline substances. The spongy mesophyll cells are few in number and they are round or oval in shape and very rich in cluster crystals of calcium oxalate. The vascular bundles that transverse these cells contain vessels which are scalariformly or spirally thickened. Alongside the midrib are found one to two layers of crystal sheathes of calcium oxalate rosettes. The lower epidermis cells are small in size, oval or almost square in their outlines and are covered with a thin faintly striated cuticle. Stomata are oval and anomocytic in type and they are quite abundant on the lower epidermis but no stomata were detected on the upper epidermis. Covering or glandular trichomes are not observed on any epidermis (DPS, ZCHRTM unpublished results).
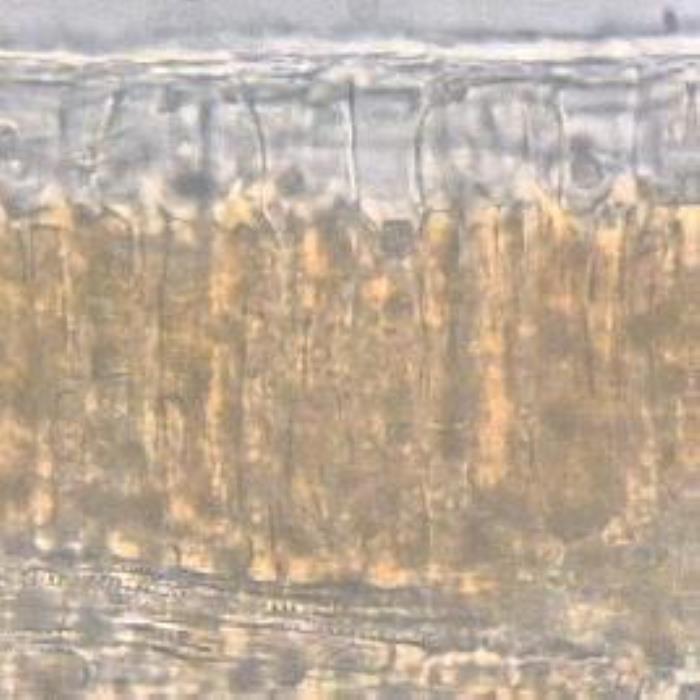
a) TS of leaf

b) Palisade cells
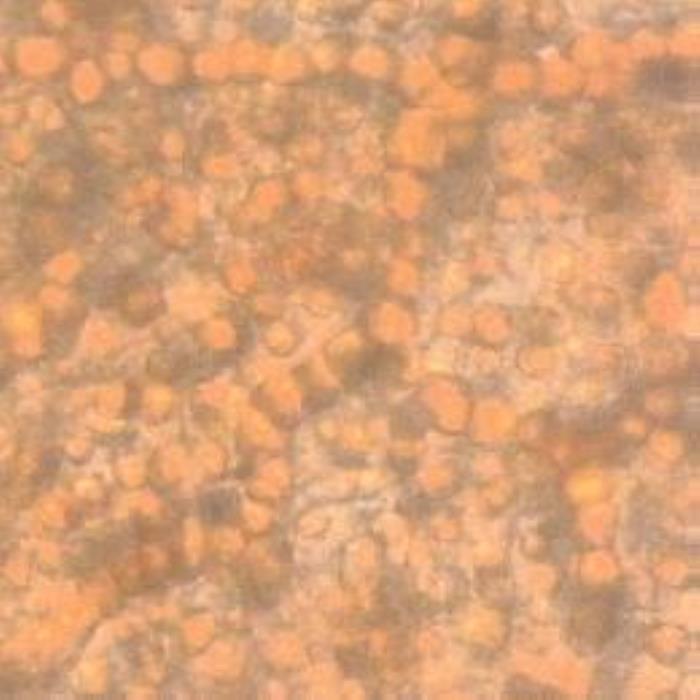
c) Spongy mesophyll
- (a). TS of the leaf showing the thin irregularly striated cuticle, the anticlinal epidermal cells, the thin two-layered orange-coloured palisade cells and the spongy mesophyll with embedded vascular bundles.
- (b). Surface view of the palisade cells that underlay the upper epidermis of the leaf.
- (c). Spongy mesophyll cells of the leaf rich in coloured mases and cluster crystals of calcium oxalate (some crystals merge together forming larger masses) (Magnification: All x 400).
Organoleptic characteristics
Appearance: Solid powder
Colour: Light green
Odour: Aromatic
Taste: Slightly bitter
Physicochemical constants
Loss in weight on drying at 105°C (%): 8.60-8.80
Solubilities ( % )
Alcohol solubility (%): 14.40
Water solubility (%): 25.60-26.80
10% ethanolic extractive (%): Not done
Ash values (%)
Total ash: 10.00
Water soluble ash: 4.00
Acid-insoluble ash: 0.80
Successive extractive (%)
Petroleum ether (60-80C): 4.35
Chloroform: 3.40
Absolute alcohol: 14.35
Distilled water: Not done
pH values
pH of 1% solution : 5.92
pH of 10% solution: 5.55
The above results are under process of publication (DPS, ZCHRTM unpublished results).
Chemical constituents
Steroids, β-sitosterol , β- D- glucoside , condensed tannins, and four saponin glycosides, namely 3-0-α- -L-fucopyranosyl (1→ 2) -P-D-glucopyranosyl (1→3) -[α- L- airabinopyranosyl] jujubogenin, 3-0-[α-D- fucopyranosyl (1→2)-β- D-glucopyranosyl -4- sulphate(1→3) -α- L-arabinopyranosyl] jujubogenin, 3-0[β-Dglucopyranosyl (1 →2) -α-L- rhamnopyranosyl (1→3)α-L-arabinopyranosyl] jujubogenin, and 3 -0[α-L- fucopyranosyl (1→2)- β-D-arabinopyranosyl (1→3)-P- Dglucopyranosyl (1→3)- α-L- arabinopyranasyl]. Jujubogenin have been isolated from the leaves. The free sugars Viz. fructose, raffinose, sucrose, glucose, galactose, rhamnose have also been isolated from different parts of the plants .(Kamil 1994; M A M etal. 1984, Ghazanfar 1999; DPS, ZCHRTM unpublished results).
Pharmacological and Toxicological studies
The antidiarrheal effects of the methanol extract of Zizyphus spina-Christi stem bark have been reported (Adzu et. al., 2003). Different extracts and fractions of the leaves, fruits and seeds of Zizyphus showed antiviral, antifungal and antibacterial activities (Shaht et. al., 2001). The effect of the aqueous extract of Zizyphus due to neuromuscular blockade. The aqueous extract of the plant is reported to possess antinociceptive activity (Adzu et. al., 2001). Zizyphus lotus (methanolic extract) was found to possess the strong mollusuicidal activity (Lahlou et. al., 2002). The inhibitory activity of the various cyclopeptides and peptide alkaloids from Zizyphus spina Christ on Ca was found to correlate well with their sedative activity (Hwang et. al., 2001).
The pharmacological and toxicological studies carried out in our laboratory and the results in brief, on Zizyphus spina-Christi (10% ethanolic extract) have been given below. The results presented without references showed unpublished data (DBMS, ZCHRTM, unpublished results).
1.) Ethanolic Extract 10%
|
ACTIVITY |
RESULTS |
|
Anti-inflammatory activity-Rat paw oedema |
The extract showed significant antiinflammatory activity which lasted for 90 to 150 min. |
|
Antinociceptive activity -Hot plate |
Extract did not show antinociceptive activity |
|
Antinociceptive activity –Writhing |
Extract showed antinociceptive activity in writhing test. |
|
Anti-diabetic activity-STZ (10% |
10% ethanolic, extract was not found effective (Zakaria et. al., 1999a; Zakaria et.al., 1999b). |
|
Anti-diabetic activity-GTT |
Glucose tolerance was improved in all experiments compared to control values (Zakaria et. al., 1999a; Zakaria et. al., 1999b). |
|
Anti-hypertension activity- |
10% extract did not have acute effect of systolic blood pressure, diastolic blood pressure and heart rate (Zakaria et. al., 2000). |
|
Locomotor activity |
Extract did not affect the locomotory parameters on sub acute treatment (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Gross behavioral studies- |
No toxic signs and symptoms observed (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Gross behavioral studies-Writhing |
No toxic symptoms observed (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Gross behavioral studies- Diarrhea, Urination |
No diarrhea and urination noticed (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Mortality |
No mortality, no toxic symptoms observed, at oral dose tested (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Motor co-ordination (String & |
Motor co-ordination not affected (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Acute toxicity studies |
No toxic symptoms observed (Islam et. al., 2000a; Islam et. al., 2000b). |
|
LD50 evaluation i.p. |
1600 mg/kg. (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Haematological studies |
Extract changes some haematological parameters (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Biochemical studied |
Extract changed some biochemical parameters (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Effect on body weight |
No significant changes in body weights recorded (Islam et. al., 2000a; Islam et. al., 2000b). |
|
Teratogenicity |
No teratogenic activity was observed; No Foetotoxicity & maternal toxicity observed |
|
Mutagenicity |
No mutagenicity (Clastogenic activity) was observed (Islam et. al., 2000a; Islam et. al., 2000b). |
Summary of the results
The 10% extract of the plant showed significant anti-inflammatory and antinociceptive activities. However, antinociceptitive activity was found significant only in writhing test. 10% extract also showed antidiabetic activity following sub acute administration. The extract failed to show any effect on blood pressure studies. There were no significant toxicological effects observed on acute administration. However, sub-acute administration daily for 90 days showed some alteration in biochemical and haematological parameters. No teratogenicity and mutagenicity were observed.
2.) Aqueous Extract
|
ACTIVITY |
RESULTS |
|
Anti-inflammatory activity-Rat paw oedema |
The aqueous extract showed significant antiinflammatory activity which lasted for 90 to 150 min. |
|
Antinociceptive activity -Hot plate |
Extract did not show antinociceptive activity |
|
Antinociceptive activity –Writhing |
Extract showed antinociceptive activity in writhing test. |
|
Anti-diabetic activity-STZ (Water extract) |
Aqueous extract is effective. Lower dose were not found effective. Only high doses are effective (Zakaria et. al., 1999). |
|
Anti-diabetic activity-STZ (Water extract) |
In sub-acute study for 5 days, the aqueous extract produced significant lowering of blood glucose (Zakaria et. al., 1999). |
|
Anti-diabetic activity-STZ (10% ethanolic extract) |
10% extract was not found effective (Zakaria et. al., 1999). |
|
Anti-diabetic activity-GTT |
Glucose tolerance was improved in all experiments compared to control values. |
|
Anti-hypertension activity - Anesthetic rats |
Aqueous extract did not have acute effects of systolic blood pressure, diastolic blood pressure and heart rate. |
|
Locomotor activity |
Extract did not affect the locomotory parameters on sub acute treatment. |
|
Gross behavioral studies-Tremor/Twitches |
No toxic symptoms observed (Islam et. al.,2001). |
|
Gross behavioral studies- writhing |
No toxic symptoms observed (Islam et. al.,2001). |
|
Gross behavioral studies- Diarrhea, Urination |
No diarrhea and urination noticed (Islam et. al., 2001). |
|
Mortality |
No toxic symptoms observed, following oral administration. (Islam et. al., 2001). |
|
Motor co-ordination (String & Platform test) |
Motor co-ordination not affected (Islam et. al., 2001). |
|
Acute toxicity studies |
No toxic symptoms were observed (Islam et. al., 2001). |
|
LD50 evaluation i.p |
1600 mg/kg. (Islam et. al., 2001). |
|
Haematological studies |
Some haematological parameters were changed (Islam et. al., 2001). |
|
Biochemical studied |
Some biochemical parameters were changed (Islam et. al., 2001). |
|
Effect on body weight |
No significant changes in body weights recorded (Islam et. al., 2001). |
|
Teratogenicity |
No teratogenic activity was observed; No Foetotoxicity & maternal toxicity observed (Islam et. al., 2001). |
|
Mutagenicity |
No mutagenicity(Clastogenic activity) observed (Islam et. al., 2001). |
Summary of results
The aqueous extract showed significant anti-inflammatory activity. The extract failed to show antinociceptive activity using hot plate method and also did not have any effect on blood pressure on acute administration. The extract was found effective in lowering of blood glucose following 5 days administration of the extract. No toxic signs and symptoms were recorded on acute administration. However, the animals showed some alteration in biochemical and haematological parameters on sub-chronic administration. No teratogenic and mutagenic effects were observed.
Antimicrobial activity
Different extracts and fraction of the leaves, fruits and seeds of Ziziphus showed antiviral, antifungal and antibacterial activities (Shahat et al. 2001).
References
- Adzu B, Amos S, Amizan MB, Gamaniel K., (2003) Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 87(2): 245-50.
- Adzu B, Amos S, Dzarma S, Wambebe C, Gamaniel K., (2002) Effect of Zizyphus spina-christi Willd aqueous extract on the central nervous system in mice. J Ethnopharmacol. 79(1): 13-6.
- Adzu B, Amos S, Wambebe C, Gamaniel K., (2001) Antinociceptive activity of Zizyphus spina-christi root bark extract. Fitoterapia. 72(4): 344-
- Alami R& AS`Ad Macksad, Medicinal Plants of Kuwait ( without date).
- Andrews, F.W. The Flowering Plants of Anglo-Egyptian Sudan; (1950&1952) vol 1+II; Arbroath, Scotland.
- Department of Biomedical Sciences, Zyed Complex for Herbal Research and Traditional Medicine, Unpublished results.
- Hwang KH, Han YN, Han BH., (2001) Inhibition of calmodulin-dependent calcium-ATPase and phosphodiesterase by various cyclopeptides and peptide alkaloids from the Zizyphus species. Arch Pharm Res. 24(3): 202-6.
- Islam, M.W., Radhakrishnan, R, Liu, X.M., Chen, H.B. , Al-Naji, M.A. Safety evaluation of Zizyphus spina-Christi L. and Teucrium stocksianum Boiss, used in traditional Medicine in the Arabian gulf. International Congress and 49th Annual Meeting of the Society for Medicinal Plant Research (Gesellschaft for Arzneipfianzenforschung), September 2-6, 2001, Erlangen, Germany.
- Lahlou M, El Mahi M, Hamamouchi J., ( 2002) Evaluation of antifungal and 5.mollusuicidial activities of Moroccan Zizyphus lotus (L.) Desf Ann Pharm Fr. 60(6): 410-4.
- Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Berghe DV, Vlietinck AJ. (2001) Chemical and biological investigations on Zizyphus spina-christi L. Phytother Res. 15(7): 593-
- Kamil M, Jayaraj A F , Ahmad F, Gunasekhar C, Samuel S, Habibullah M and Chan K. Pharmacognostic Protocols for Standardisation of Zizyphus spina-christi. Journal of Pharmacy and Pharmacology,1999 (51) Supp:226.
- Ghazanfar S A. Handbook of Arabian Medicinal Plants. CRC Press, p.182, 1994.
- Batanouny,K.H. et al, Wild Medicinal Plants in Egypt: an Inventory to Support Conservation and Sustainable Use. (1999) Palm Press, Egypt, ISBN 977 5089 24 7.
- El Ghazali, Gamal, E. B et al. Medicinal Plants of Sudan, Part I.(1986) Khartoum Univ. Press.
- El-Ghonemy, A. A. Encyclopedia of Medicinal Plants of the United Emirates. (1993) 1st Edition, University of UAE.
- Ghazanfar, S. A. Handbook of Arabian Medicinal Plants.(1994) Library of Congress.
- Jongbloed, M.V. The Comprehensive Guide to the Wild Flowers of the united Arab Emirates, Erwda, (2003) Emirates Printing Press, Dubai, U.A.E.
- Kamil M., A.F. Jayaraj, F. Ahmad, C. Gunasekhar, S. Samuel, M. Habibullah and K. Chan. Pharmacognostic protocols for standardisation of Zizyphus spinachristi. Journal of Pharmacy and Pharmacology, (1999 ),51,Supp. 226.
- Kotb, T. F. Medicinal Plants in Libya.(1985) Arab Encyclopedia House. Tripoli-Libya.
- Mandaville, J. P. Flora of Eastern Saudi Arabia. (1990) Kegan Paul International Ltd. England.
- Miller A.G., Morris M. Plants of Dhofar, The southern Region of Oman: Traditional, Economic and Medicinal Uses.(1987) Office of the Advisor for conservation of the Environment, Sultanate of Oman.
- Western, A. R. The Flora of United Arab Emirates, an introduction. (1986) Publication of the UAE University.
- Zakaria, M.N.M., M.W. Islam, R. Radhakrishnan, A. Ismail, M. Habibullah and K. Chan Antidiabetic properties of Zizyphus spina-christi in STZ- diabetic mice. “2000 Years of Natural Products Research – Past Present and Future”, Amsterdam, July 26-30 1999.
