
Capparis cartilaginea /كبر
Capparis galeata Fresen Capparis spinosa var. galeata (Fres.) Hook. f. & Thorns. Capparis uncinata Edgew.
Qubar, Kubar, Shflah, Lusef, Aslub, Shafih, Qateen, Felfel aljabl, Shalem, Salbo
Caper plant, Cartilage Caper
Kubr, Sediru, Ashflah,
Capparaceae

Aerial parts

Herbarium specimen
Ethnobotanical Characteristics
Description
Prostrate or scrambling shrubs, sometimes hanging from the rocks, glabrous, glaucous, often fleshy with short, crooked branches. Leaves petiolate with an ovate blade, broadly elliptic or orbicular, 2-6 cm long, 1.5-6 cm broad, entire, more or less fleshy, drying sub coriaceous; apex entire or slightly emarginated with usually hooked-yellowish brown spine inserted below the apex. Flowers axillary and solitary, asymmetrical, large, usually, white; pedicel stout, 4-5 cm long, increasing up to 8 cm (rarely more) and thickened in fruit. Sepals asymmetrical, posterior one about twice as large as the rest, deeply galeate, up to 4 cm long and 1-1.5 cm broad towards the apex. Petals unequal, not exceeding the largest sepal, posterior pair somewhat hooded and enclosed in the hooded posterior sepal. Stamens many, about 3 cm long. Gynophore 3-4 cm long, increasing up to 6 cm in fruit and becoming stout. Fruits ovoid or ellipsoid, 3-5 cm long, 2-3 cm broad, often reddish and ribbed, many seeded and pulpy (eFloras, Jongbloed, 2003, Mandaville, 1990).
Habitat and Distribution
North and Equatorial East Africa, Arabia, Iraq, South Iran and West Pakistan. The plant is widespread in U.A.E. Scattered locations in the Hajar Mountains. Widespread in the Ru'us al-Jibal. This is primarily a coastal species, and prefers rocks and hillocks as its habitat.
Part(s) Used
Leaves, bark, flowers, fruits, roots and whole plant.
Traditional and Medicinal Uses
The fruits and seeds are reportedly edible. Stems, bark, infructescences and roots are food additive. Leaves: are known for their carminative, aphrodisiac, anti-inflammatory, antirheumatic properties, also for childbirth, paralysis, toothache, gum inflammation, tumors, and swellings. The dried leaves are chewed against cough. Fruits: used as diuretic, carminative, aphrodisiac, anti-inflammatory, stimulant, also used for toothache, gum inflammation, arteriosclerosis and renal problems. Bark: used as a tonic, astringent, purgative, appetizer, has anti-rheumatic and anti-diarrhea properties. Flowers: used as diuretic, expectorant and stimulant. Whole plant: used as a diuretic, carminative, anti-inflammatory and antiseptic. Leaves and stems are crushed in water and heated; for bruises, snakebites, swelling and headache, and on skin rash to reduce itching. Seeds: used for feminine problems and gastric ulcer. Roots: used as a tonic, astringent, diuretic and for tumors and swellings (El-Ghonemy, 1993; Kotb, F.T. 1985; Batanouny, 1999; Hamed, 2007; Gilani, 1994; Miller, A.G. et al., 1988; Ghazanfar, 1994; Thulin, 1993).
Pharmacognosy and Phytochemistry
Plant material Studied
Dried leaf
General appearance
The leaf is yellowish to light green in colour. It is ovate, petiolate, thick, and leathery tipped with a blunt spine while the margin is entire to sinuate. Leaves vary in length and size.
Microscopic characteristics
Leaf: The surface view of both epidermises show that they are composed of polygonal cells, though rectangular cells are also found in the lower epidermis. Few oval stomata are seen. A transverse section of the leaf shows that it is isobilateral, and the two surfaces have thick striated cuticle and both upper and lower epidermises are free from covering or glandular trichomes. The upper epidermis consists of oblong cells with thick, shiny cell walls underlain by a layer of palisade tissues that consist of short compactly packed oblong cells with wary cell walls. These are somewhat rich in different glistening crystalline masses which resemble calcium oxalate cluster crystals, but calcium oxalate crystals have not been detected in the leaf. The palisade tissue layer is underlain by many rounded, polygonal and oblong spongy mesophyll cells; the polygonal oblong cells, which are relatively large, occupy a large zone at the central part of the leaf and they contain few crystalline masses. Some of them embed few fibro-vascular tissues, which are comparatively large with spirally thickened vessels and short, broad tracheids having thick walls with characteristic pits, and they are close to each other. The area between the polygonal oblong cells zone and the short celled palisade layer adjacent to the lower epidermis is occupied by layers of compact rounded cells which are very rich in the crystalline masses. The palisade tissues and the zones of the compact spongy rounded cells close to each epidermis show dark areas of rich cell contents. Some idioblasts are occasionally observed between epidermal cells, and they are filled with crystalline matter.
Powdered plant material
The powder is pale yellow to beige in colour and it is a homogeneous fine powder. The mesophyll cells contain relatively large amounts of rosettes of different sizes of a pale green substance, especially in the spongy mesophyll cells near to palisade layers. Some spongy mesophyll cells have pitted cell walls. Vessels are annularly and spirally thickened. Idioblasts are detected.
Parts studied
leaf
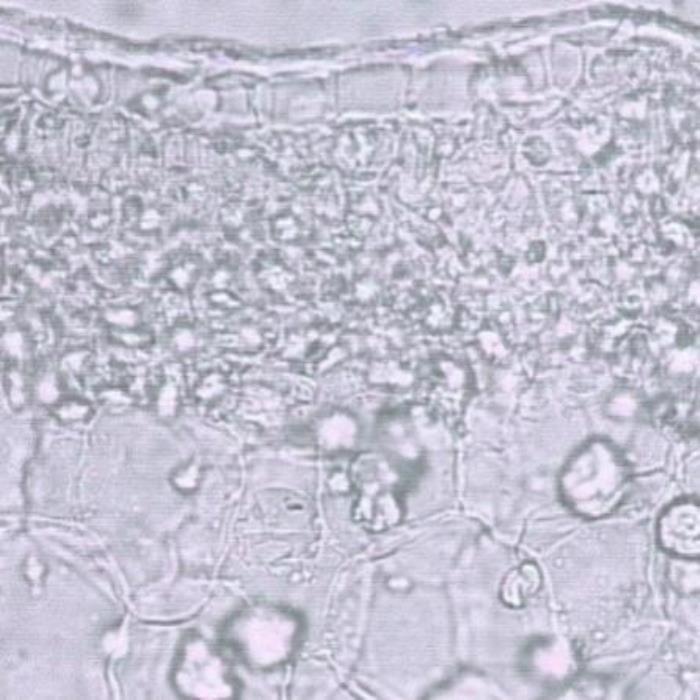
A) TS of leaf
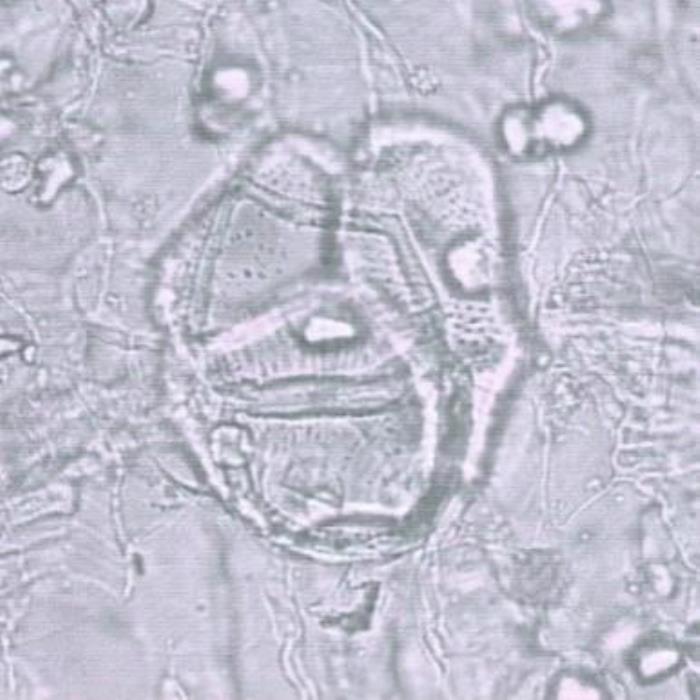
B) Pitted vessels
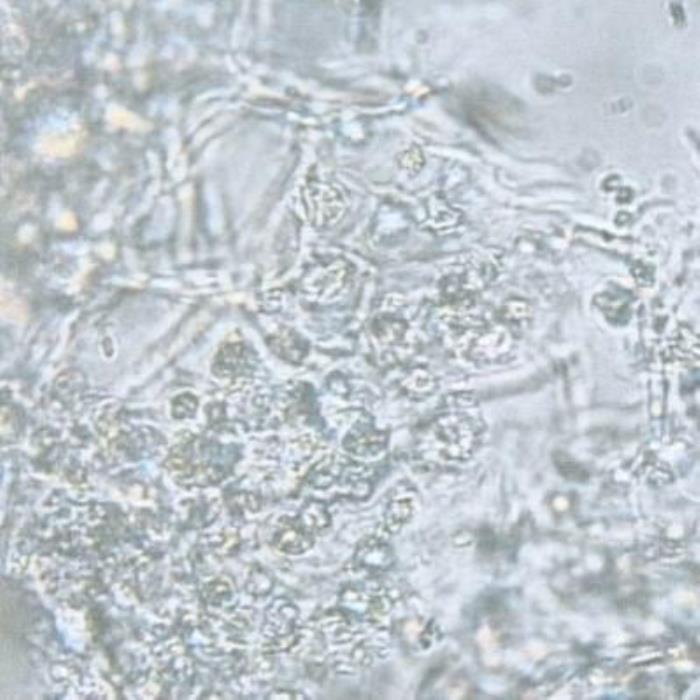
C) Mesophyll cells
- A. TS of a leaf at midrib region. Upper epidermal cells with thick cell walls covered by a thick cuticle and underlain by compact layers rich in contents of crystalline and amorphous masses; the upper layer just beneath the epidermis seems to be palisade reduced in size but the majority of the cells are rounded or prolonged.
- B. A transverse section of the leaf at the midrib. A group of pitted vessels joined together to form a rounded structure surrounded by parenchymatous spongy mesophyll cells and vascular tissues (magnified).
- C. The mesophyll cells are rich in crystalline substances with characteristic shapes.
Chemical constituents
The fruit contains vitamin C, proteins and carbohydrates (Ghazanfar, 1994). Four flavonoids: kaempferol-3-O-rutinoside, quercetin-3-O-rutinoside, quercetin-7-O-rutinoside and quercetin-3-) -glucoside-7-O-rhamnoside. Four isothiocyanates: butyl isothiocyanate, 6-methylsulphonylhexyl isothiocyanate, 7-methylsulphonylheptyl isothiocyanate, 5-benzylsulphonyl-4-pentenyl isothiocyanate. Main flavonoidal compound (rutin) (Ahmed, 2007). Leaves contains: alkaloids, sterols, triterpenes, flavonoids and tannins (Awatif, 2001).
The following chemical studies have been carried out (Quality Control methods, 1998; Evans, 1996) on the aerial part of the plant Capparis cartilaginea (ZCHRTM unpublished work).
Physicochemical Constants
Loss of weight on drying at 105°C: 10.00
Absolute alcohol solubility: 1.60
Water solubility: 40.00
Successive extractives (%)
Petroleum ether (60-80°C) : 0.90
Chloroform : 1.10
Absolute alcohol : 4.00
Water soluble : 41.05
Ash values (%)
Total ash : 26.00
Water soluble ash : 8.99
Acid insoluble ash (10% HCl) : Nil
pH values (aqueous solution)
pH of 1% solution : 5.441-5.483
pH of 10% solution : 5.123-5.138
Elemental analyses
Ash values (British Herbal Pharmacopeia)
Assay and identification of metal (AOAC International)
|
Apparatus |
AA-6800 Shimadzu-Flame method |
||||
|
Element |
Std. conc. µg/ml (ppm) |
Sample conc.mg/ml |
samples absorbance |
Actual conc.mg/ml |
Actual conc. (%) |
|
Cr |
1, 2, 4 |
20.02 |
0.0004 |
0.00261 |
0.0002611 |
|
Zn |
0.5, 1, 2 |
20.02 |
0.1097 |
0.05257 |
0.005257 |
|
Cu |
0.5, 1, 2 |
20.02 |
0.0366 |
0.0174600 |
0.00174600 |
|
Fe |
1, 2, 4 |
20.02 |
0.0555 |
0.10639 |
0.010639 |
|
K |
1, 2, 4 |
20.02 |
1.9667 |
1.1129 |
0.11129 |
|
Pb |
1, 2, 4 |
20.02 |
0.000 |
0.000 |
0.000 |
|
Cd |
0.25, 0.5, 1 |
20.02 |
0.000 |
0.000 |
0.000 |
UV Spectral studies
|
Ultraviolet Spectrum (USP reference) |
||||
|
Apparatus |
Milton Roy Spectronic Genesys 5 Spectrophotometer - Milton Roy. |
|||
|
Sample conc. (mg / ml) |
Solvent |
λ max (nm) |
λ min (nm) |
Abs.( λ max - λ min) |
|
1.082 |
Intestinal Fluid simulated without pancreatic pH=7.50.1 |
268 |
250 |
0.281 – 0.263 |
|
5.07 |
Gastric Fluid simulated without pepsin pH =1.20.1 |
no shift |
no shift |
- |
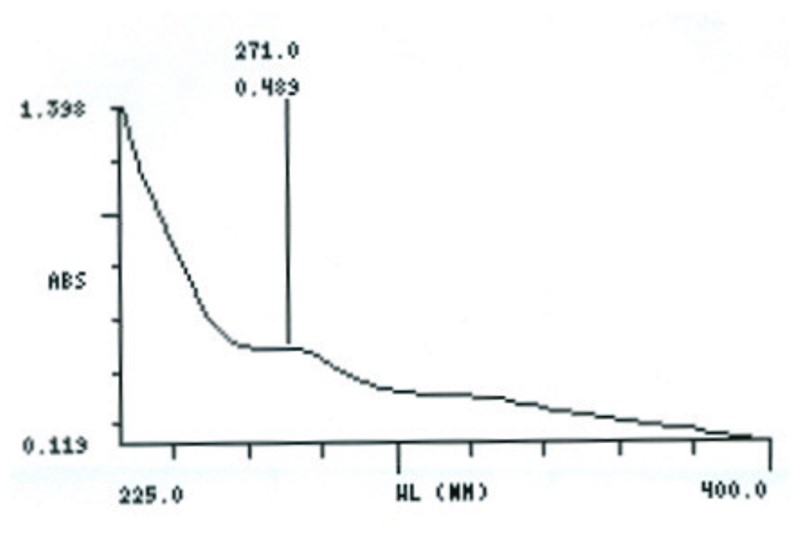
Intestinal Fluid simulated without pancreatic
Chromatographic Studies
Thin layer chromatography (Wagner and Bladt, 1996)

A
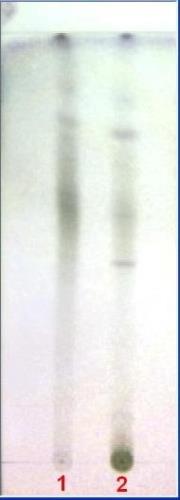
B

C

D
TLC fingerprint of Pet. ether (60-80) °C extract (track 1) and MeOH extract (track 2)
|
Mobile phase Fig. |
A&C |
: |
Ethyl acetate, methanol, water (100:13.5:10) |
|
|
B |
: |
Toluene, ethyl acetate (54:1) |
|
|
D |
: |
Toluene ethyl acetate (93:7) |
|
Detection |
A |
: |
UV 366 nm |
|
Derivatization |
B,C&D |
: |
Vanillin-Sulphuric acid –vis. |
Pharmacological and Toxicological studies
Aqueous extract
This plant, common to the Mediterranean basin. has The traditional medicine practitioners have used it for its diuretic and antihypertensive effects and also in certain pathological conditions related to uncontrolled lipid peroxidation. The plant is known to counteract the harmful effects induced by IL-1 beta. This protection appeared to be greater than that elicited by indomethacin, which is usually given in joint diseases. Since plant possesses a chondroprotective effect, it might be used in the management of cartilage damage during the inflammatory processes (Panico, 2005).
Repeated oral administration of the aqueous extract of Capparis sp. was studied showed that a dose of 20mg/kg on lipid metabolism in normal and streptozotocin-induced diabetic rats and reduced lipid content in both, normal and severe hyperglycemic rats (Eddouks, 2005).
Capparis is one of the ingredients of Liv-52, a famous drug used for treating liver cirrhosis. The results demonstrated that the patients treated with Liv-52 for six months had significantly decreased ascites, and serum ALT, and AST. This protective effect of Liv-52 is attributed to the diuretic, anti-inflammatory, anti-oxidative, and immunomodulation properties of the component herbs (Huseini, 2005). Two lyophilized, methanolic extracts obtained from Capparis spinosa flowering buds carried out at room temperature, were investigated for their allergic and antihistaminic nature. These two extracts displayed marked antiallergic effectiveness (Trombetta, 2005).
Capparis sp. has been reported to possess antioxidant activity (Germano, 2002). The aqueous extract of Capparis showed significant anti-inflammatory activity against carrageenan induced oedema in rats. The butanol fractions of both Capparis deserti and Capparis cartilaginea showed no antioxidant activity. The results are expressed as radical scavenging activity (Ghazanfar, 1994). Capparis spinosa plant was tested for their antipyretic, analgesic, and anti-inflammatory activity (Ageel, 1986).
The ethanol and water extracts of Capparis zeylanica leaves showed dose-dependent and significant increases in pain threshold using tail-immersion test (Ghule, 2007).
Administration of Capparis decidua fruit extract significantly reduced serum total serum cholesterol, LDL cholesterol, triglycerides and phospholipids reflecting its hypolipidemic potential (Purohit, 2005). Capparis decidua may have potential use as an antidiabetic agent and in lowering oxidative stress in diabetes (Yadav, 1997).
An ethanolic extract of Capparis cartilaginea at a dose of 1-10 mg/kg b.w. caused a dose-dependent decrease in blood pressure and heart rate in anaesthetized rats (Anwar, 2006).
The anti-allergenic properties of lyophilized extracts of Capparis spinosa showed protective effect against the bronchospasm showing marked anti-allergenic effectiveness (Trombetta, 2005). Pre-treatment with water extract of Capparis zeylanica augmented the humoral immune response to sheep red blood cells preventing myelosuppression in mice treated with cyclophosphamide drug (Ghule, 2006). Antiviral and immunomodulatory properties of methanol extract of Capparis spinosa buds, rich in flavonoids, including several quercetins and kaempferol glycosides, improved immune surveillance of a virus infection by up-regulating the peculiar proinflammatory cytokines (Arena, 2007).
Capparis spinosa reportedly has antihepatotoxic activity in isolated rat hepatocytes, in in-vitro technique (Gadgol, 1999).
Antioxidant activities of the bioactive components were studied on the plant extract. The findings suggest the use of Capparis sp. with foods contributes to oxidizable lipids and also protects against the oxidative stress-based diseases (Tesoriere, 2007).
Extracts of Capparis cartilaginea showed moderate antimicrobial activity against two gram-positive bacteria, (Staphylococcus aureus and Enterococcus faecalis) and two gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), and yeast, Candida albicans, in a qualitative agar diffusion test (Alasbahi, 1999).
The following pharmacological and safety evaluation studies were carried out on the plant Capparis cartilaginea (Aqueous extract) (Derelanko 2002; Han, 2003):
|
ACTIVITY |
RESULTS |
|||
|
Strong |
Moderate |
Mild |
Negative |
|
|
Analgesic |
√ |
|
|
|
|
Anticonvulsant |
|
|
√ |
|
| Antidiarrheal effect (Castor oil - induced diarrhea) | √ | |||
|
Effect on rabbit jejunum |
|
|
√ |
|
|
Effect on rat fundus |
|
|
√ |
|
|
Effect on Guinea pig ileum |
|
√ |
|
|
|
Effect on Guinea pig tracheal chain |
|
|
|
√ |
|
Effect on right rat atria |
|
√ |
|
|
|
Antithrombotic effect |
√ |
|
|
|
|
Studies on biochemical parameters |
√ |
|
|
|
|
Studies on hematological parameters |
√ |
|
|
|
| Acute toxicity in mice | √ | |||
|
Motor co-ordination (grip strength & motor activity |
|
|
|
√ |
|
Rectal temperature |
|
|
|
√ |
|
Body weight |
|
|
|
√ |
|
Mortality |
|
|
|
√ |
Summary of the results
The results of the present study revealed that the plant extract showed positive analgesic activity, decreased prothrombin time and increased the fibrinogen level and cardio tonic activity.
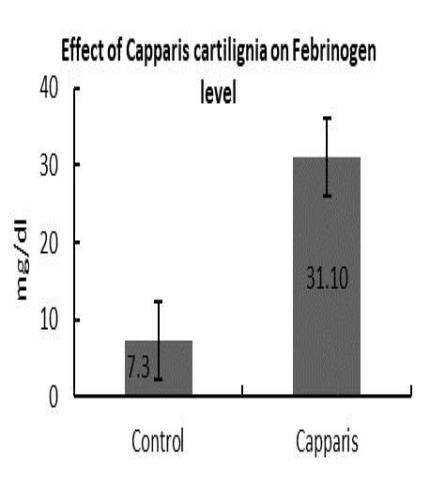
Effect on Fibrinogen level
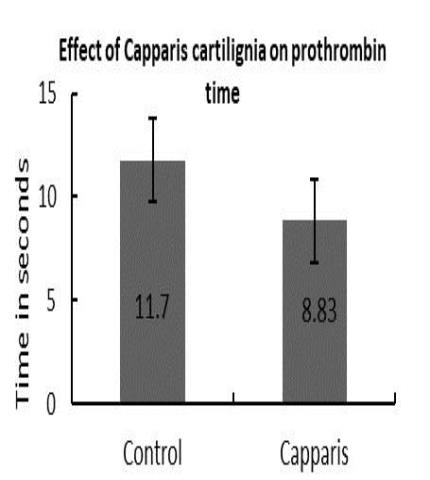
Effect on prothrombin time
The plant showed a mild antidiarrheal activity and mild effect against PTZ induced convulsions. The plant extract did not show Bronchiodilatory effect. No overt changes recorded in the biochemical profile compared to control values. The plant extract was found relatively harmless on acute administration. The LD50 of the drug was found to be > 5g/kg (route- gastric intubation).
Antimicrobial activity
The aqueous extract of the whole plant did not inhibit the growth of Mycobacterium smegmatis, C. tropicalis, different strains of Methicillin Resistant Staphylococcus aureus, different strains of ESBL-producing K. pneumonia, E. coli, Pseudomonas aeruginosa.
References
- Ageel AM Al-Yahya MA, Al-Said MS, Tariq M. Parmar NS, Mossa JS, (1986). Anti-inflammatory activity of some Saudi Arabian medicinal plants. Agents Actions. 17: 383-384.
- Alasbahi H, Fullbright L, Safiyeva S, and Craker LE. (1999). Antimicrobial Activity of Some Yemeni Medicinal Plants. Journal of Herbs, Spices & Medicinal Plant, 6: 75 - 83.
- Anwar ul H, Gilani, K A. (2006). Hypotensive and spasmolytic activities of ethanolic extract of Capparis cartilaginea. H. E. J. Research Institute of Chemistry, Pakistan.
- Arena A Bisignano G, Pavone B, Tomaino A, Bonina FP, Saija A, Cristani M, D'Arrigo M, Trombetta D. (2007). Antiviral and immunomodulatory effect of a lyophilized extract of Capparis spinosa L. buds. Phytother Res. 3: 1272-1278.
- Batanouny, K. (1999). Wild medicinal plants in Egypt. Cairo, Giza, Egypt: Dept. of Botany, University of Cairo.
- Bauer AW, Kirby WMM, Sheriss JC, Turck M. (1966) Antibiotic susceptibility testing by standardized single method. Am J Clin Pathol; 45:493–6.
- British Herbal Pharmacopoeia (1996).4th Ed.: British Herbal Medicine Association (BHMA). Derelanko, M. J., & Hollinger, M. A. (2002). Hand book of toxicology. (2nd ed.). Boca Raton, USA: CRC Press.
- Eddouks M, Lemhadri A, Michel JB. (2005). Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J Ethnopharmacol, 98: 345-350.
- Elegami, A. A., Almagboul, A. Z., Omer, M. E. A., & El Tohami, M. S. (2001). Sudanese plants used in folkloric medicine: screening for antibacterial activity. Part x., Fitoterapia, 72(7), 810- 17.
- El-Ghonemy, A.A. (1993). Encyclopedia of medicinal plants of the United Arab Emirates. (1st ed.). Abu Dhabi, UAE: United Arab Emirates University.
- Evans, W.C (1996).Trease and Evans’ Pharmacognosy,(14th ed,p.105 )Saunders ,London.
- Flora of Pakistan. e-floras, Web.
- Gadgoli C, Mishra SH. (1999). Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J Ethnopharmacol, 66: 187-192.
- Germano MP, De Pasquale R, D'Angelo V, Catania S, Silvari V, Costa C. (2002). Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem, 50: 1168-1171.
- Ghazanfar, S. (1994). Handbook of Arabian medicinal plants. Florida, USA: CRC Press.
- Ghule BV, Murugananthan G, Nakhat PD, Yeole PG. (2006). Immunostimulant effects of Capparis zeylanica Linn. Leaves. J Ethnopharmacol, 108: 311-5.
- Ghule BV, Murugananthan G, Yeole PG. (2007). Analgesic and antipyretic effects of Capparis zeylanica leaves. Fitoterapia, 78: 365-369.
- Hamed , A.R., Abdel-shafeek, K.A., Abdel-Azim , N.S., Ismail , S.I, & Hammouda , F.M. (2007). Chemical investigation of some capparis species growing in Egyp and their antioxidant
- activitiy, E-CAM, S1.(4), 25-28. Retrieved from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2206244/
- Han , J., & Hoosier, G. L. V. J. (2003). Hand book of laboratory science, animal models. (Second ed., Vol. II). USA: CRC Press.
- Huseini HF, Alavian SM, Heshmat R, Heydari MR, Abolmaali K. (2005). The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomedicine, 12:619-624.
- Jongbloed, M., Feulner, G., Boer, B., & Western, A. (2003). The comprehensive guide to the wild flowers of the United Arab Emirates. Abu Dhabi, UAE: ERWDA.
- Kotb, F. (1985). Medicinal plants in Libya. Beirut, Lebanon: Arab Encyclopedia House.
- Mandaville, J. (1990). Flora of Eastern Saudi Arabia. London, UK.: Kegan Paul International Ltd.
- Miller, A, and M Morris. (1988). Plants of Dhofar - the Southern region of Oman. Oman: The office of the advisor for conservation of the environment, Diwan of Royal court.
- Mothana R A A, Abdo SA ,Hasson S, Althawab FM, Alaghbari SA, Lindquist U.( 2008) Antimicrobial, antioxidant and cytotoxic activity and phytochemical screening of some Yemeni medicinal plants. Evid Based Complment Alternat Med. 7(3):323-30.
- Official Methods of Analysis of AOAC International (1999).16th. Ed.Vol.I and II
- Panico AM, Cardile V, Garufi F, Puglia C, Bonina F, Ronsisvalle G. (2005). Protective effect of Capparis spinosa on chondrocytes. Life Sci, 77:2479-288.
- Purohit A, Vyas KB. (2005). Hypolipidaemic efficacy of Capparis decidua fruit and shoot extracts in cholesterol fed rabbits. Indian J Exp Biol, 43: 863-866.
- Quality control methods for medicinal plant materials (1998).World Health Organization, Geneva.
- Tesoriere L, Butera D, Gentile C, Livrea MA. (2007). Bioactive components of Capparis spinosa L. from Sicily and antioxidant effects in a red meat simulated gastric digestion, J Agric Food Chem, 55: 8465-8471.
- Thulin, M. (1993). Flora of Somalia. (Vol.1). Kew, UK: Royal Botanic Gardens, Kew
- Trombetta D, Occhiuto F, Perri D, Puglia C, Santagati NA, De Pasquale A, Saija A, Bonina F. (2005). Antiallergic and antihistaminic effect of two extracts of Capparis spinosa L. flowering buds. Phytother Res, 19: 29-33.
- Yadav P, Sarkar S, Bhatnagar D. (1997). Action of Capparis decidua against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res, 36: 221-228.
- Wagner, H. Bladt, S.(1996). Plant Drug Analysis-A Thin layer Chromatography Atlas, (2nd Ed.) Springer-Verlag, Berlin Heidelberg.
- ZCHRTM unpublished work
- محمد العودات, (1988), جورج لحام النباتات الطبية واستعمالاتها، الجزء الأول-الطبعة الثانية.الأهالي، سوريا
